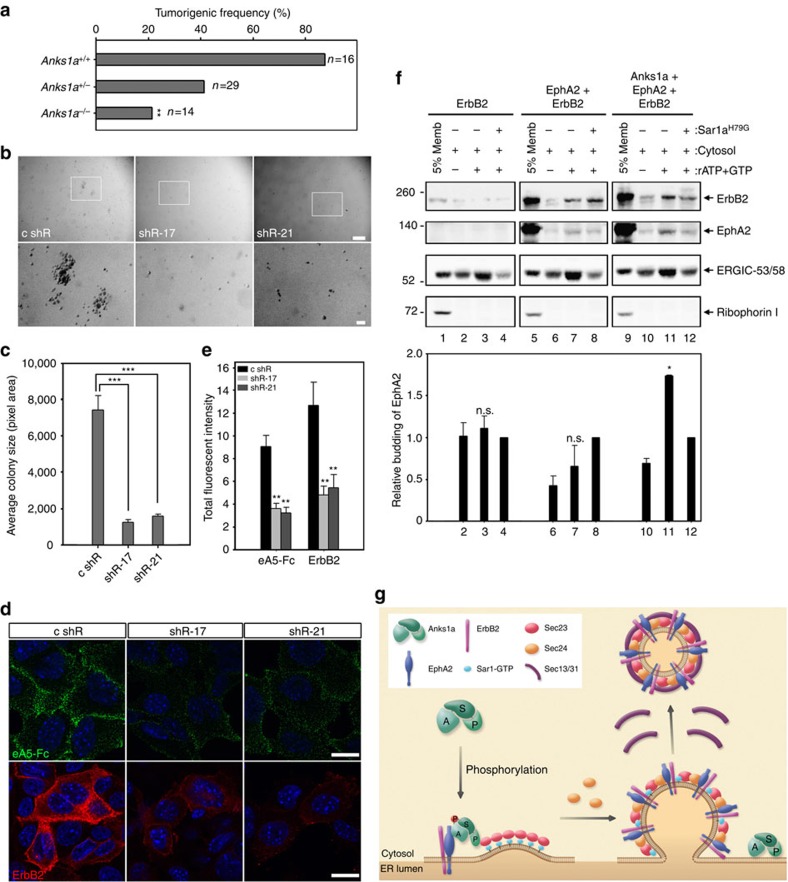
Figure 5. Anks1a influences breast tumorigenesis by regulating the ER export of EphA2–ErbB2 complexes.
(a) Anks1a+/− female mice were crossed with Anks1a+/−; MMTV-Neu male mice to obtain female mice with the indicated genotypes. All experiments monitoring breast tumour formation were carried out in mice of the FVB genetic background (n=16, 29, 14 per genotype). **P<0.01, χ2-test. (b,c) PMTCs were isolated from breast tumours of MMTV-Neu female mice and transduced with Anks1a-specific shRNA lentiviruses. Infected cells were subjected to soft agar colony formation assays as described in Fig. 4e. Scale bar, 250 μm (top panel); 50 μm (bottom panel). ***P<0.001, Student's t-test. (d,e) PMTCs were stained with ephrinA5-Fc or an anti-ErbB2 antibody without permeabilization to detect cell surface receptors as described in Fig. 1c. Scale bar, 20 μm. **P<0.01, Student's t-test. (f) HEK293 cells were co-transfected with the indicated constructs and COPII vesicle-budding assays were performed as described in Fig. 2f (n=4). Note the use of Sar1aH79G as a control for the normalization of the EphA2 budding. *P<0.05, one-way analysis of variance test. (g) Model in which Anks1a facilitates the export of EphA2 and ErbB2 RTKs from the ER. In mitogen-activated cells, Ser-647 and Ser-663 of Anks1a are likely the major regulatory phosphorylation sites. Our experiments indicate that serine phosphorylation of Anks1a is critical for its subcellular localization to the ER. Serine phosphorylation may induce a conformational change in Anks1a so it can interact effectively with both EphA2 and Sec23 in the ER. The ANK domain of Anks1a binds to EphA2 or EphA2/ErbB2 complexes, whereas the PTB domain binds to Sec23, a critical component of COPII vesicles. Then, other COPII components (that is, Sec24 and Sec13/31) are recruited to Sec23 in the ERES, possibly competing away Anks1a. This dynamic COPII biogenic process would direct the loading of RTK cargos into growing COPII carrier vesicles. n.s., not significant. Data represent means±s.e.