Fig. 1.
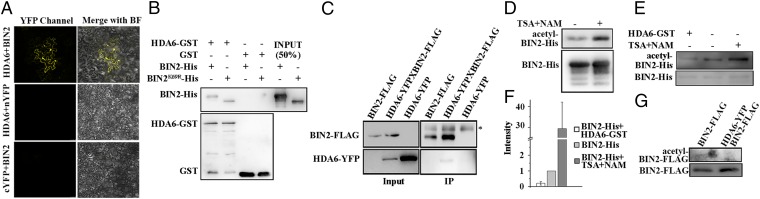
HDA6 interacts with and deacetylates BIN2. (A) HDA6 interacts with BIN2 in BiFC assays. The nYFP-BIN2 or nYFP and HDA6-cYFP or cYFP constructs were cotransformed into the pavement cells of N. benthamiana. BF, bright field; magnification, 20×. (B) HDA6-GST interacts with BIN2-His in GST-pull down assays. (C) HDA6-YFP coimmunoprecipitated by BIN2-FLAG in plants. The asterisk indicates a nonspecific band. (D) The recombinant BIN2-His protein purified from E. coli was acetylated. The E. coli strain containing the BIN2-His construct was cultured in LB medium with or without 5 nM TSA and 5 mM NAM. The acetylation level of the purified BIN2-His was detected with an anti–acetyl-Lys antibody. (E) BIN2-His can be deacetylated by HDA6 in vitro. The acetylation status of BIN2-His was determined with an anti–acetyl-Lys antibody. (F) Statistical analysis of the relative acetylation level of BIN2-His. (G) BIN2-FLAG is acetylated in plants. BIN2-FLAG and HDA6-YFP BIN2-FLAG (crossed from BIN2-FLAG and HDA6-YFP) plants were grown on soil for 2 wk. The acetylation level of the immunoprecipitated BIN2-FLAG protein was detected with an antiacetyl-Lys antibody. Error bars represent SE.