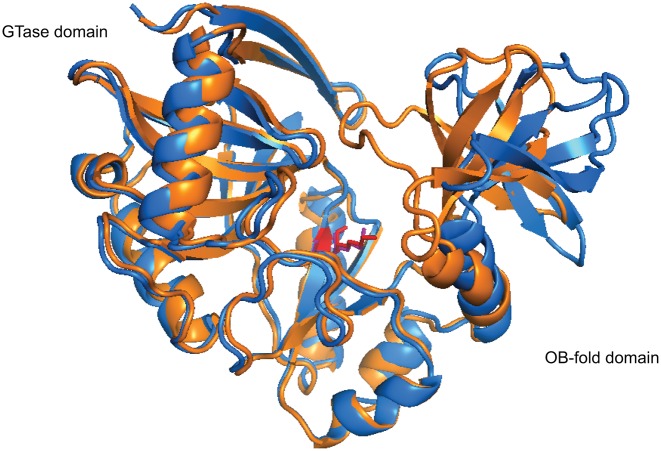
Figure 4.
The opened and closed conformations of Chlorella virus PBCV-1 capping enzyme (PDB 1CKM). The PBCV-1 RNA capping enzyme adopts a bilobed structure where the N-terminal guanylyltransferase domain and C-terminal OB fold domain sit on either side of the protein with the active site situated at the interface of the two domains. The asymmetric unit of the crystal structure of Chlorella virus capping enzyme contains two protein units, each exhibiting a different conformation. In the opened conformer (blue), the cleft between the two domains is ∼15 Å at its widest, whereas in the closed conformer (orange), the cleft closes one end off to the solvent completely due to rigid movement of the OB-fold domain. Interestingly, Lys82, the nucleophile that forms the lysyl-Nζ-linkage with incoming GMP (red and violet), shows very limited movement within the active site (100).