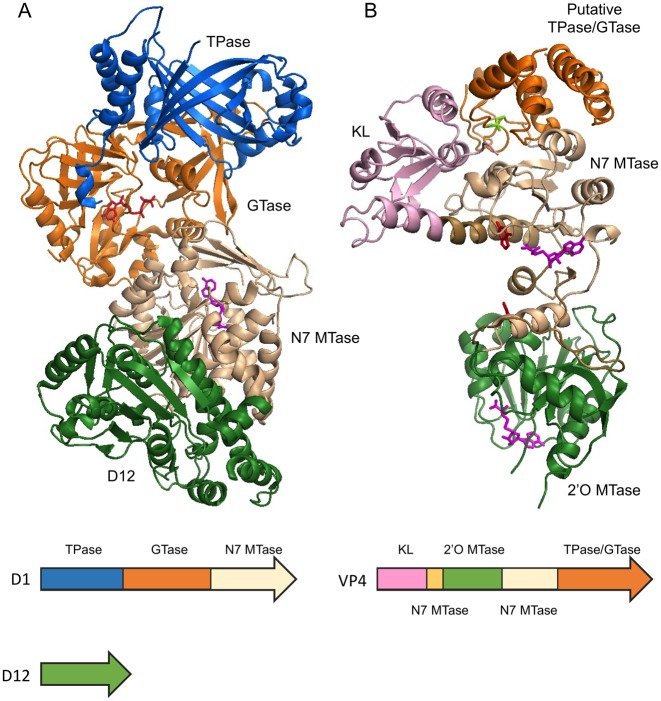
Figure 5.
Multifunctional RNA capping enzymes. (A) Vaccinia capping enzyme structure co-crystallized with GTP and SAH (PDB 4CKB). The enzyme composes of two subunits D1 and D12. The functional domains are ordered as TPase (blue), GTase (orange) and guanine-N7 MTase (beige) from N- to C-terminus in D1. D12 subunit is colored in green. Note the β-barrel characteristic of triphosphate tunnel metaloenzymes (TTM). As indicated by the SAH molecule (magenta), the guanine-N7 MTase active site opens to the back, away from the GTase active site as indicated by the GTP molecule (red). (B) Bluetongue virus capping enzyme VP4 structure. The crystal form that contains two guanine molecules (red) and two SAH molecules (magenta) is shown (PDB 2JHP). The functional domains are arranged from N- to C-terminus in the order of kinase-like (KL) domain (pink), 2′O MTase domain (green), guanine-N7 MTase and the putative combined TPase/GTase domain (orange). The guanine-N7 MTase domain is composed of two discontinuous sequences which are colored in light brown and beige. Two stretches of polypeptide of 10 and 13 amino acid residues within the C-terminal TPase/GTase domain are missing in the structure. The SAH molecules (magenta) clearly identify the active sites of the guanine-N7 MTase and 2′O MTase in the structure. The putative TPase catalytic residue Cys518 is colored in bright green.