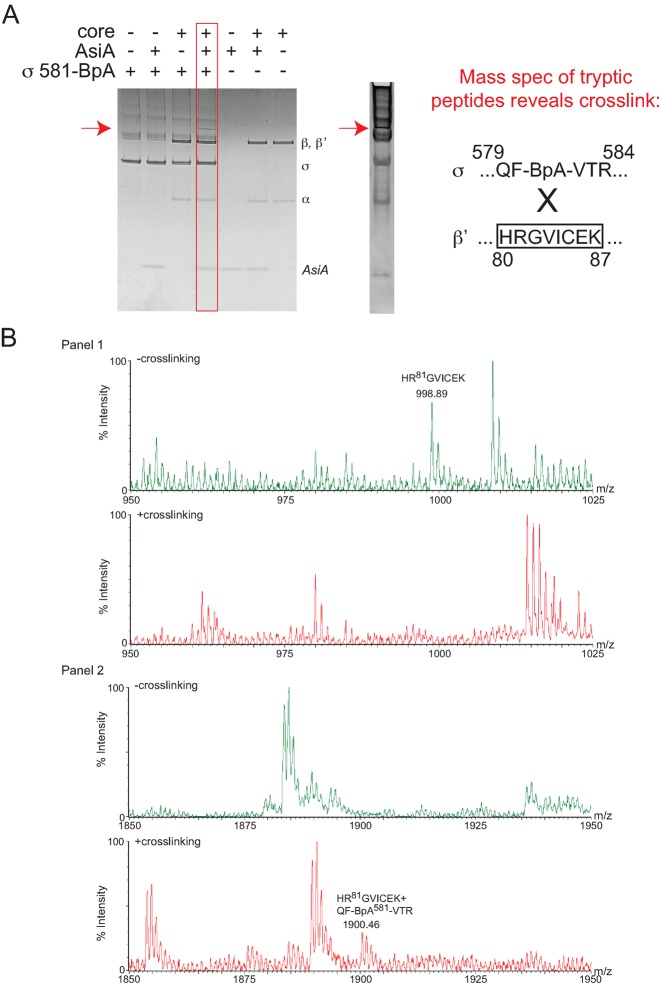
Figure 3.
σD581BpA photocrosslinks to the region of β’ containing residues 80–87. (A) Left, Coomassie-stained 10–20% Tris-tricine gel showing the species obtained after UV treatment of a solution containing the indicated proteins. The slowly migrating band observed in the presence of σD581BpA, core, and AsiA is indicated by the red arrow. Right, Coomassie-stained gel showing the species removed for digestion with typsin, followed by MALDI-TOF mass spectrometry. The sequence of the identified crosslinked peptide between σ and β’ is shown. (B) Mass spectra showing crosslink formation between σD581BpA and β’. (Panel 1) Mass spectra of the tryptic digests of the non-crosslinked proteins (top) and crosslinked forms (bottom) from m/z = 950–1025. In the non-crosslinked spectrum, the mass of the β’ peptide 80HRGVICEK87 is labeled; this ion is not observed upon crosslinking. (Panel 2) Mass spectra of the tryptic digests of the non-crosslinked proteins (top) and crosslinked forms (bottom) from m/z = 1850-1950. In the crosslinked spectrum, a unique ion with m/z = 1900.46 is noted. This mass is consistent with that expected for β’ 80HRGVICEK87 crosslinked to sigma 579QF-BpA-VTR584.