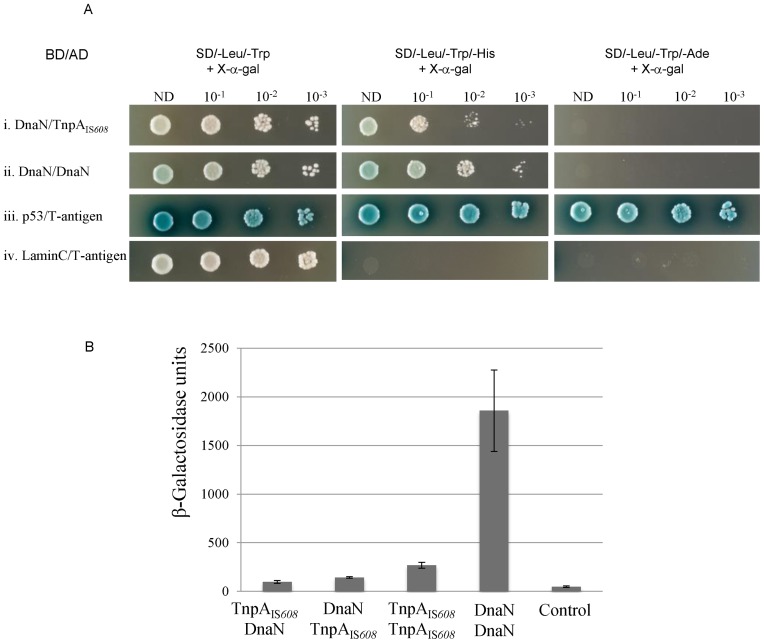
Figure 3.
Interaction of TnpAIS608 with DnaN in yeast and bacterial two-hybrid systems (BACTHs). (A) Yeast two-hybrid system. For each hybrid, dilutions (10−1, 10−2 and 10−3) of yeast cell cultures normalized (OD = 2) for each interaction and expressing both bait and prey constructs were spotted on several selective media. ND: non-diluted; BD: binding domain; AD: activation domain. Positive (p53/T-antigen) and negative (LaminC/T-antigen) controls are shown at the bottom of the figure. (B) BACTH. DnaN/TnpAIS608 and TnpAIS608/DnaN correspond to configurations with fused proteins T25-DnaN/TnpAIS608-T18 and T25-TnpAIS608/DnaN-T18 respectively (28). The efficiency of functional complementation between the indicated hybrid proteins was quantified by measuring β-galactosidase activities in Escherichia coli BTH101 carrying the corresponding plasmids as described in ‘Materials and Methods’ section. The figure represents the mean and standard deviation of at least six independent assays.