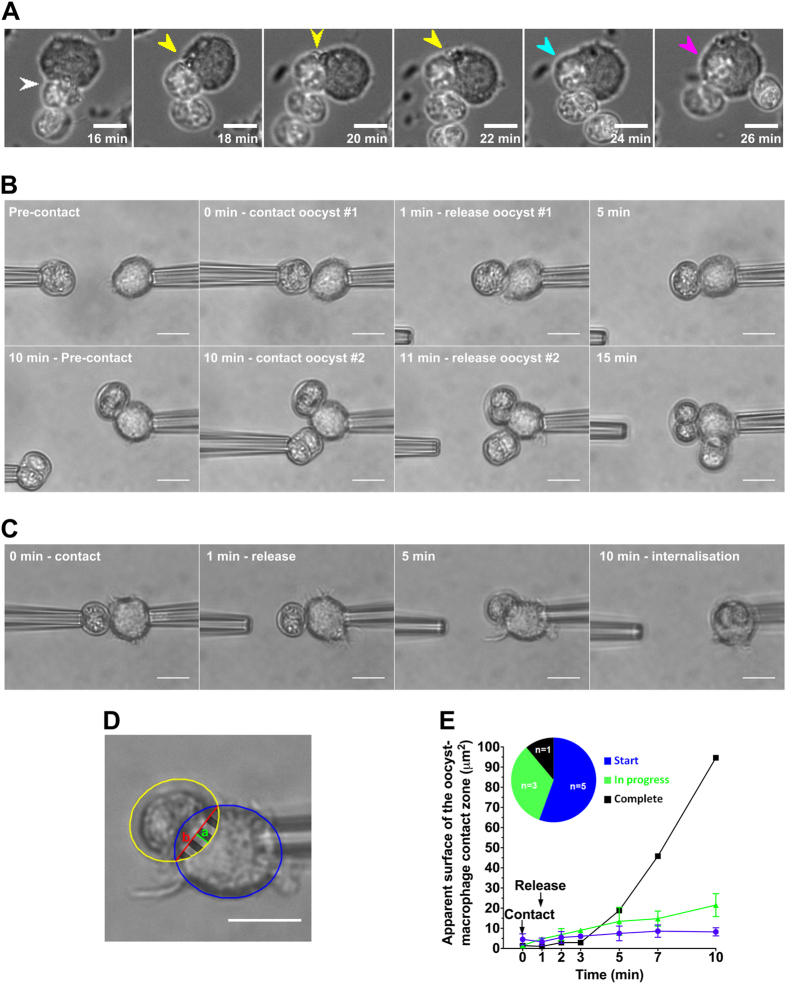
Figure 1.
Analysis of initial interactions between RAW macrophages and native T. gondii oocysts recorded by time-lapse video microscopy at 37 °C (A) and by using micropipette techniques at room temperature (B–E). (A) Selected frames recapitulating the different steps of the oocyst internalisation by macrophage cells: oocyst adhesion (white arrowhead), macrophage membrane extension (yellow), closure of the phagocytic cup (blue), and full internalisation (magenta). Here, the process starts at t = 16 min, i.e. 16 min after introducing oocysts in the macrophage cell culture (at this time, after sedimentation, most of oocysts located in the same focal plane as the macrophage cells). Scale bar: 10 μm. (B) After one minute of contact, a first oocyst adhered to a macrophage and processed by it (i.e. moved around). A second oocyst was presented to the macrophage at t = 10 min, released at t = 11 min, and was also processed by the macrophage. Scale bar: 10 μm. (C) Follow-up of the complete internalisation of one oocyst by a micropipette-held macrophage. Scale bar: 10 μm. (D) The positions of the macrophage and the oocyst were recorded by elliptical blue and yellow overlays respectively. The apparent surface of the contact zone (striped area, A) was calculated by measuring the respective length of the segments a (green) and b (red) and then by using the following formulae: 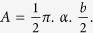 Scale bar: 10 μm. (E) Plot of the apparent surface of the oocyst-macrophage contact zone over time.
Scale bar: 10 μm. (E) Plot of the apparent surface of the oocyst-macrophage contact zone over time.