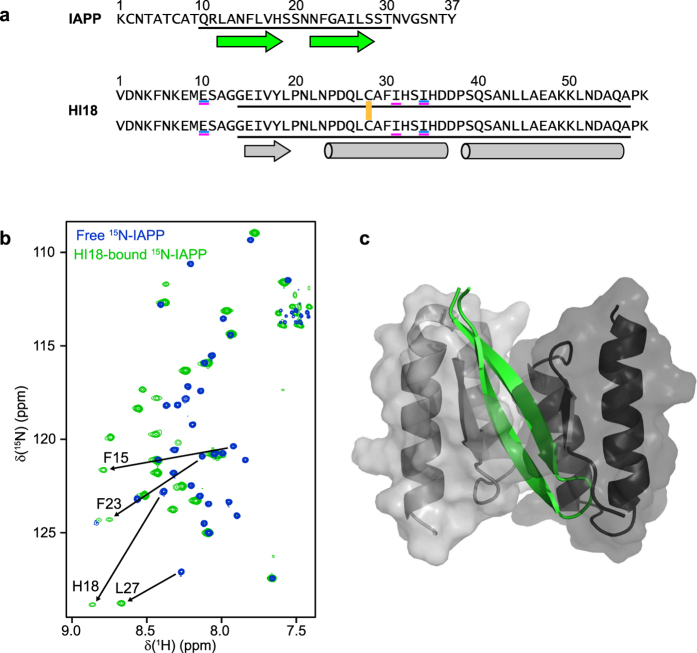
Figure 1. Sequences and topology of the IAPP:HI18 complex.
(a) Amino acid sequences of IAPP and HI18. The two subunits of HI18 are linked by a disulfide bond involving the Cys-28 residues (yellow). Residues that are exchanged in HI18 compared to ZAβ3 and AS10 are underlined in blue and magenta, respectively. The segments that constitute the folded core of the IAPP:HI18 complex are underlined in black. The positions of α-helical and β-sheet secondary structure in the complex are indicated by cylinders and arrows, respectively. (b) (1H–15N)-HSQC NMR spectra of [U-13C,15N]-IAPP in the absence (blue) and presence (green) of a 20% molar excess of [NA]-HI18. Selected resonances experiencing large changes in chemical shifts upon binding are highlighted. (c) Ribbon drawing of the IAPP:HI18 complex. Residues 10–30 of IAPP are shown in green. Residues 13–56 of the two HI18 subunits are shown in light and dark gray with semi-transparent surface display.