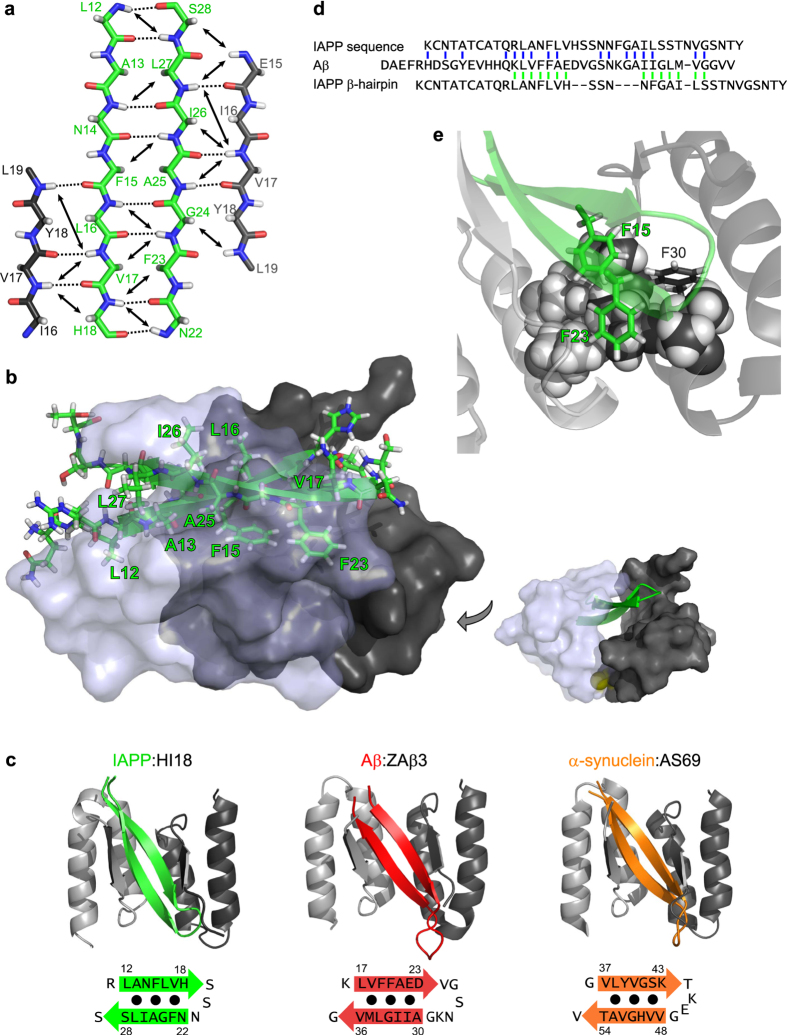
Figure 2. β-Hairpin conformation of IAPP bound to HI18.
(a) Scheme of the β-sheet registry in the IAPP:HI18 complex. β-Strand backbones of IAPP (green) and the two HI18 subunits (light and dark grey) are displayed in straight extended conformation for clarity. Long-range HN-HN and HN-Hα NOE connectivities defining the β-sheet registry are represented by arrows. Backbone hydrogen bonds identified by PyMOL are shown as dotted lines. (b) Ribbon and stick representation of the IAPP β-hairpin (green) interacting with HI18. The two HI18 subunits are shown in semi-transparent and non-transparent surface representation, respectively. Hydrophobic residues in the IAPP β-hairpin are labelled. The sulfur atoms constituting the disulfide bond between the HI18 subunits are displayed in yellow in the image on the right. (c) Comparison of the β-hairpin of IAPP (green) bound to HI18 with β-hairpins of Aβ (red) and α-synuclein (orange) bound to the related binding proteins ZAβ3 (PDB:2OTK) and AS69 (PDB:4BXL), respectively. (d) Sequence alignment of IAPP and Aβ based on sequence similarity32,33 (top, blue) or based on the positions of the β-strands in the HI18- and ZAβ3-complexes (bottom, green). (e) Aromatic-aromatic and aromatic-hydrophobic interactions in the core of the IAPP:HI18 complex. Phe-15 and Phe-23 of IAPP are located in the central core of the complex where they are in contact with each other. One face of both Phe-15 and Phe-23 packs against an extended hydrophobic interaction surface composed of the side chains of Leu-27, Ile-31, and Ile-34 (displayed as spheres) of the two HI18 subunits.