Abstract
Acute antibody mediated rejection (AMR) is recognized as a major cause of graft loss in renal transplant recipients. Early acute AMR in the first few days after transplantation occurs primarily in sensitized renal transplant recipients with donor-specific alloantibody at the time of transplant and is a relatively “pure” form of acute AMR. Late acute AMR occurs months to years after transplantation and is commonly a mixed cellular and humoral rejection. While there is no consensus regarding optimum treatment, we contend that rational therapeutic approaches are emerging and the acute episode can be managed in most instances. However, new therapies are needed to prevent ongoing chronic injury in these patients.
Keywords: antibody mediated rejection, transplant glomerulopathy, positive crossmatch transplantation, renal transplantation, kidney transplant
Introduction
In the early days of renal transplantation, the major problem associated with donor-specific alloantibody (DSA) was hyperacute rejection. With more sensitive techniques to identify DSA and increased understanding of the histological changes associated with DSA, it became clear that a form of acute rejection that was distinct from cellular rejection was possible in renal transplant recipients. This was termed acute antibody mediated rejection (AMR). With decreasing rates of acute cellular rejection, acute antibody mediated rejection has emerged as a major cause of graft loss in the weeks and months after transplantation.
Here, we review our current understanding of acute AMR including current clinical management at our institution. We also discuss the possible connection between acute and chronic AMR and outline gaps in our understanding of both of these vexing entities. Finally, we examine specific therapeutic modalities and the evidence of their utility in the management of acute AMR.
Early and Late AMR—Two Different Clinical Entities
It is important to understand that there are really two distinct clinical settings that are termed acute AMR and their treatment may vary slightly Table 1. The first clinical setting is early AMR which occurs in the first few days few days to weeks after transplantation. Early AMR most commonly occurs in allosensitized recipients (i.e. those with known DSA at the time of transplant), though it can occur rarely in patients with no DSA at transplant. The incidence varies with the amount of DSA present at the time of transplantation. In patients with high levels of DSA (i.e. sufficient to cause strongly positive crossmatch) the incidence may be as high as 40% in the first month after transplantation, while the incidence is less than 10% in patients with a negative crossmatch and DSA demonstrated only by solid phase assay[1].
Table 1.
Early versus late acute AMR
| Early AMR | Late AMR | |
|---|---|---|
| Timing | Days to weeks post-transplant | Months to years post-transplant |
| Pathophysiology | Levels of preformed DSA increase from memory B-cell response following antigen stimulation |
Formation of De Novo DSA or increase
in preformed DSA in setting of suboptimal immunosuppression and/or concomitant cellular rejection |
| Histology | C4d+ peritubular capillaries
on immunofluorescence, acute tubular necrosis, peritubular capillaritis, and glomerulitis |
Similar to early AMR in most
cases: Peritubular capillaritis and glomerulitis +/− C4d positivity in setting of interstitial inflammation and tubulitis. Features of transplant glomerulopathy may also be present. |
| Treatment | Plasmapheresis IVIG Eculizumab Bortezomib Rituximab |
Treatment of cellular rejection (ex.
steroids and anti-lymphocyte therapy). Consider plasmapheresis and IVIG if DSA MFI > 6000. In absence of transplant glomerulopathy, eculizumab, bortezomib, or rituximab could be considered. |
Early AMR in this setting is relatively easy to identify since it is usually a “purer” form in which cellular rejection is commonly absent. The recipient usually demonstrates a relatively rapid rise in serum creatinine level (usually day 10-14 after transplantation) and the biopsy shows the classic signs of AMR including C4d+ staining of the peritubular capillaries on immunofluorescence and other features of injury including acute tubular necrosis, microvascular inflammation (peritubular capillaritis and glomerulitis). More severe forms might show mesangiolysis and glomerular microthrombi. Serum levels of DSA are elevated due to a combination of preformed antibody and newly-formed antibody from memory responses. In our series, a B flow cytometric crossmatch >360 (corresponding to a Mean Fluorescence Intensity (MFI) of roughly 9000) in the early post-transplant period was almost always associated with an early AMR episode [2].
Early AMR can be quite severe and is a major cause of early graft loss. Thus, we recommend aggressive, early treatment in most cases. We caution that while it is easy to attribute increases in serum creatinine to dehydration or an elevated tacrolimus level, in a highly-sensitized patient 10 days after transplantation, early AMR should be the leading diagnosis. Therefore, we may obtain the biopsy and draw the blood for serum DSA measurements, then begin plasma exchange (PE) therapy before these results return. In this way, we treat preemptively and can stop therapy if the diagnosis of AMR is not confirmed.
PE is our first line of therapy and is sufficient to decrease serum DSA levels and thus to reverse most cases of early AMR (1). Seven to 10 days of PE are commonly needed and our goal is to reduce DSA levels to a B FXM <200 or an MFI <4000. The biopsy findings of AMR may persist for several days after the DSA levels have dropped. Thus, we tend to tailor therapy to DSA levels and not the biopsy findings.
In more severe forms of early AMR, the serum DSA levels and the serum creatinine may continue to rise despite daily PE. These cases of early AMR are at highest risk for graft loss and require more aggressive treatment. While there is no consensus in the field regarding the best treatment for these severe cases, we would add eculizumab (1200 mg initially, then 600 mg after every PE) to the treatment regimen. Terminal complement blockade with eculizumab appears to significantly block ongoing graft damage and protects the graft until DSA levels begin to respond to PE [3]. After 7 days of PE/eculizumab therapy, we reassess and may discontinue eculizumab is DSA levels are decreasing and if there is clinical improvement. As described below, other groups have advocated the use of splenectomy, bortezomib, rituximab and/or high dose intravenous immunoglobulin (IVIG). However, given the heterogeneity and rarity of these severe cases of early AMR, no controlled studies are available and recommendations are mainly based on few cases.
In highly-sensitized patients with an expected high incidence of early AMR, prevention may be a more prudent approach. For example, our group has shown that in patients with a BFXM channel shift >200 but less than 450 at baseline, the incidence of early AMR was 41% using a PE-based regimen [1]. When eculizumab was added this regimen at the time of time of transplantation and continued for at least 1 month, the incidence of early AMR was only 7.7% [3]. In addition, the few early AMR episodes that occurred were easily treated with PE and none required splenectomy. Eculizumab was able to be discontinued in half of the patients at 1 month because DSA levels remained low. Graft survival at 1 year was 100% in the eculizumab group versus 98% in the PE-alone group (unpublished).
While DSA levels will return to low levels (ex. MFI <3000 or so) in most patients after treatment of early AMR, some patients with have persistently high DSA levels. Despite having received intensive therapy and multiple PE treatments, these patients are at high risk for the development of chronic AMR and accelerated graft loss. There likely is no effective therapy for these patients. Our data suggests that continuation of eculizumab does not prevent chronic injury. Our current approach would be to discontinue therapy and then reassess them at approximately 3 months after transplantation. At this point, if the patient shows evidence of chronic AMR (ex. microvascular inflammation and persistent DSA), we would treat with bortezomib (1.3 mg/BSA x 2 cycles [8 doses]), followed by PE x 7 days and then high dose IVIG (2 g/kg x1). Unfortunately, this approach rarely results in dramatic improvement in either DSA levels or histology and clearly new, more effective therapies are needed.
Late Acute AMR
Acute AMR also can be diagnosed months to years after kidney transplantation. In this clinical setting, an acute elevation in serum creatinine develops and a history of non-adherence to immunosuppression may commonly be elicited. A biopsy shows the Banff ′97 histologic features associated with acute AMR (C4d+ peritubular capillaries and evidence of histologic injury) and serum DSA level are present. However, histologic features of acute cellular rejection also may be present (interstitial inflammation and tubulitis) [4]. In this setting, the production of DSA is likely due to the concomitant acute T cell response to the allograft [5].
One of the major decisions to be made in these patients is whether or not the acute AMR is truly contributing to graft dysfunction. In our program, we treat the cellular component of the late acute AMR with either corticosteroids or anti-T cell antibody, depending on the severity of the cellular rejection and the magnitude of graft dysfunction. If the DSA levels are relatively low (ex. MFI < 2000 or so), we may elect not to treat the antibody component of this rejection episode as DSA levels may decrease when the T cell rejection is treated.
In contrast, when DSA levels are high (ex. MFI > 6000), we postulate that the DSA is truly contributing to graft dysfunction and merits treatment. We treat this episode similar to an early acute AMR episode employing multiple PE therapies as our first-line therapy. In contrast to early acute AMR, most cases of late acute AMR are mild and easily controlled by a combination of anti-T cell therapy and PE. The major problem that quickly emerges is that graft function does not completely return to baseline and evidence of chronic injury develops. Indeed, many patients have evidence of chronic injury (interstitial fibrosis and even transplant glomerulopathy) at the time of diagnosis of late acute AMR. Thus, the distinction between acute and chronic injury is somewhat blurred. Again, the clinical setting dictates treatment based on our prediction of outcome. For example, a rapid rise in DSA with an allograft biopsy showing few chronic changes might be reversible and thus might benefit from eculizumab therapy if PE is unsuccessful. In contrast, a small rise in DSA with an allograft biopsy showing extensive chronic changes (ex. Banff ′97 cg 3 lesions) might not benefit from any therapy.
Chronic AMR
A detailed discussion of chronic AMR is beyond the scope of this review. However, a few points regarding the role of acute AMR in chronic AMR deserve mention. First, the histology of acute and chronic AMR overlaps significantly and it is unclear whether or not the pathobiology of the two are truly distinct. Acute AMR has been shown to be a major risk factor for the development of chronic AMR. However, our recent eculizumab trial clearly shows that prevention of early AMR has little impact on the incidence of chronic AMR [3] Figure 1. Thus, early clinical AMR may be critically dependent on C5, but chronic injury can occur without it. Antibody mediated injury that does not require C5 might include: direct activation of endothelial cells by DSA and microvascular inflammation (either vie c3a-mediated chemotaxis, recognition by the Fcγ receptor of macrophages and NK cells, or other mechanisms) [6, 7]. We recently examined the long-term outcomes of these patients and found that persistently high DSA levels appeared to be the greatest risk factor for chronic injury.
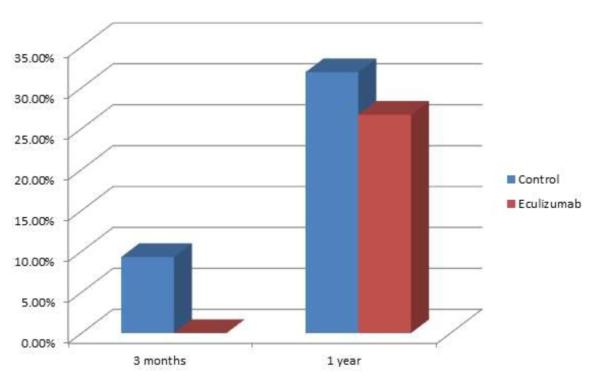
Fig 1. Chronic AMR in the absence of acute AMR in eculizumab-treated patients.
At 3 months post transplant, no patients treated with eculizumab had transplant glomerulopathy (Banff cg score > 0), while this was present in 9.3&% of control patients. At 1 year, transplant glomerulopathy was present in 31.9% of control patients and 27.0% of eculizumab treated patients p=0.62.
Despite this, the treatment of acute AMR and how it is treated could affect the incidence of subsequent chronic AMR. Studies to examine this link more clearly are needed.
Specific Therapies for acute AMR
Now that we have described some of the various clinical presentations of acute AMR and have presented our programmatic approaches, we believe that it is important to acknowledge that there are numerous other rational therapies for this problem Table 2. We will discuss the details of each and any published data supporting their use.
Table 2.
Therapeutic options for acute AMR
| Therapy | Studies | Mechanism of Action | Effectiveness |
|---|---|---|---|
| Plasmapheresis | Bonomini et al. (1985)[37] Kirubakaran et al. (1981)[38] Allen et al. (1983)[39] Blake et al. (1999)[40] Stegall et al. 2006[41] |
Physical removal of antibody |
Temporarily effective at reducing antibody level depending on continued antibody production. |
| Intravenous Immunoglobulin (IVIG) |
Glotz et al. (1993)[42] Tyan et al. (1994)[43] Jordan et al. (1998)[11] Casadei et al. (2001)[13] Tyan et al. 1994 [43] Stegall et al. 2006 [41] Lafaucheur et al. (2009)[44] |
Multiple immunomodulatory actions |
Variable efficacy |
| Splenectomy | Locke et al. 2007 [17] Tzvetanov et al. 2012[18] |
Reduce B-cell and Plasma Cell burden |
Variable efficacy |
| Rituximab | Kaposztas et al. 2009[45] Lafaucheur et al. 2009 [44] |
CD 20+ inhibitor leading to reduction in B-cells |
Variable efficacy |
| Bortezomib | Everly et al. 2008[26] Walsh et al. 2010[27] Wong et al. 2010[28] |
Proteasome inhibitor leads to plasma cell apoptosis |
Variable efficacy |
| Eculizumab | Stegall et al. 2011[3] |
Terminal complement (C5) inhibitor |
Very effective at inhibiting acute AMR, but does not prevent chronic AMR. Expensive. |
Understanding the basic immunology of antibody production and antibody-mediated rejection is imperative in understanding the various treatment approaches for antibody mediated rejection and their limitations. Furthermore, an understanding of the basic biology of the rejection is needed for therapy development.
Plasmapheresis
Plasmapheresis is the physical removal of antibodies, and was the main treatment of antibody mediated rejection for many years. The effect of plasmapheresis tends to be temporary until the source of antibody is controlled. Marked variability exists in the efficacy plasmapheresis from 0% to 93%[8]. Randomized control trials have not confirmed a benefit from plasmapheresis alone [9], but this therapy remains a first line therapy for AMR.
Intravenous immunoglobulin (IVIg)
Intravenous immunoglobulin (IVIg) is polyvalent IgG antibodies that comes from a pool of over a thousand donors and comes in a variety of formulations. It has been FDA approved for many autoimmune conditions and is approved for acute antibody mediated rejection and desensitization for transplant recipients. The precise mechanism of the immunomodulatory action of IVIg is largely unknown. However, IVIg is thought to saturate the Fc receptors on macrophages, suppress the production of inflammatory mediators, modulate complement, interfere with DSA binding and/or activity, and suppression idiotypic antibody [10].
No randomized controlled trials for using IVIG in AMR have been published to my knowledge. However, several groups have reported their experience using IVIG as treatment for acute AMR and as desensitization prior for highly sensitized transplant candidates [11-14]. This therapy along with steroids and plasmapheresis was the mainstay of treatment until newer agents such as rituximab became available. Currently IVIG is often used as adjunctive therapy for AMR combined with plasmapheresis and newer biological agents. The dose used for AMR and desensitization are typically higher than that used for immunodeficiency at 1-2mg/kg as a single dose or as multiple low dose infusions of 100mg/kg after plasmapheresis. IVIg is typically well tolerated. High dose therapy can sometimes lead to volume overload. Other complications include renal failure (typically from sucrose containing solutions), headaches, and thrombotic complications [15]. Additionally, because chromatographically derived IVIg products contain antibody blood group antibodies, IVIg has been associated with temporary hemolytic anemia [16].
Splenectomy
Although invasive, splenectomy is an underappreciated potential therapy for acute antibody mediated rejection. Splenectomy is associated with rapid recovery of renal function, especially in patients otherwise refractory to conventional treatments [17]. Six of eleven patients with acute AMR refractory to IVIG and plasmapheresis, had improved renal function 2 weeks following splenectomy [18]. Normally the spleen is rich in mature B-cells and not plasma cells, but spleens removed at times of acute antibody mediated rejection exhibit a distinct increase in CD 138+ plasma cells as compared to spleens removed for other reasons [19]. Another group found that in addition to increased CD 138+ plasma cells, the size of T and B lymphocyte aggregates were decreased [20]. Based on these findings, high antigenic burden might induce the spleen to sequester plasma cells or enable rapid differentiation of B cells [19]. Splenectomy should be considered in patients refractory to other therapies, especially with the minimization of surgical and infectious risks with laparoscopic techniques and vaccination. The role of splenectomy on future chronic antibody mediated rejection is unknown.
Rituximab
Rituximab is a monoclonal antibody directed against CD 20, a marker widely expressed on B-cells from early pre-B cell stage of development to the mature B-cell. Binding of the Fc portion of rituximab to the CD 20 leads to antibody dependent cellular cytotoxicity, complement-dependent cytotoxicity, and a rapid 70-80% decline in the B cell population. Other mechanisms of rituximab have been proposed in the rheumatology literature. Rituximab might down regulate CD40, the costimulatory molecule needed to augment the interaction between T and B cells, or inhibit cytokines interfering with B-cell proliferation and differentiation [21].
In a nonrandomized retrospective study, patients treated with rituximab in addition to plasmapheresis and IVIG had improved graft and renal function as compared to those treated with plasmapheresis and IVIG alone.
Unfortunately the response to rituximab appears to be variable. Some studies have shown significant benefit, while others are less encouraging. Rituximab is likely ineffective in circumstances of high antibody burden because the established short or long lived plasma cells producing the antibody lack CD20. The variable expression of CD20 on B cells might also play a role in its variable efficacy [22].
Bortezomib
Inhibiting the plasma cell to ultimately reduce antibody secretion is a logical therapeutic step in treating antibody mediated rejection. Bortezomib is a proteosome inhibitor that leads to apoptosis by inhibiting the degradation of abnormal and misfolded proteins. Proteasome inhibition has other immunomodulatory effects as well. Plasma cells are especially vulnerable to apoptosis with this therapy, and bortezomib has become one of the main treatments for multiple myeloma. It has been theorized that high protein or antibody production in states such as multiple myeloma may increase susceptibility to proteasome inhibition [23]. Our group has shown that in vitro, bortezomib causes apoptosis of human plasma cells preventing alloantibody production [24]. This effect has not been demonstrated with the use of other major therapeutics in transplantation including thymoglobulin, rituximab, or IVIG. In vivo, our group has also found the bortezomib lead to a reduction in plasma cells in the bone marrow of highly sensitized transplant candidates, but this did not translate into a reduction in donor specific antibody [25]. However, it is possible that this therapy potentiates the effectiveness of plasmapheresis. We found a greater reduction in DSA levels with plasmapheresis in those treated with bortezomib as compared to historical control patients treated with plasmapheresis alone [25].
No randomized clinical trials using bortezomib for AMR exist, but several clinical series have been published. In the first report of bortezomib use in AMR, eight episodes of AMR in six patients were treated with bortezomib alone after being refractory to other common treatments including plasmapheresis, IVIg, thymoglobulin, and rituximab. This treatment was associated with a > 50% reduction in immunodominant DSA, stabilized to improved renal function, and improved renal histology [26]. This group also published their experience using bortezomib as first line therapy for early AMR in 2 patients combined with plasmapheresis and rituximab. Both patients experienced a rapid reversal of AMR and elimination of DSA within 14 days [27]. The START collaborative (Strategic Anti-humoral Therapies in Renal Transplantation) used a bortezomib based regimen for AMR in 96 kidney, heart, and pancreas transplant recipients. The results from this collaboration have only been published in abstract form, but suggest that bortezomib may also be associated with a reduction in DSA.
Unfortunately, not all groups have found bortezomib therapy as successful in treating AMR [28-30]. [31]. Four patients with sub-acute AMR and persistent DSA were treated with 1 cycle of bortezomib and DSA levels were unchanged or higher over follow-up up to 270 days[29, 30]. Regardless of the variable efficacy of bortezomib, this therapy appears to be well tolerated when 1-2 cycles are given [32]. The main adverse effects include anemia, thrombocytopenia, peripheral neuropathy, and gastrointestinal effects.
The inconsistent results reported after the use of bortezomib is likely related to the diversity of AMR in general. As mentioned earlier, a spectrum of AMR exists and it may or may not be associated with cellular rejection. The plasma cell population generating alloantibody leading to AMR may be very different in patients with late AMR in the setting of cellular rejection or noncompliance versus the early AMR that occurs as part of the memory response of a presensitized patient. In fact, a greater reduction in immunodominant DSA has been shown after treatment with proteasome inhibition in early AMR as compared to late AMR [5].
The impact of proteasome inhibition may be related to the differential survival of the distinctive plasma cell populations including the plasmablast, the short-lived plasma cell, and long-lived plasma cell. The plasmablast is essentially a B-cell undergoing differentiation to ultimately become a short-lived plasma cell. These short-lived plasma cells are the most common of the antibody-producing cells and usually only survive a few days in the spleen or inflammatory sites at the time of immunization or infection [33]. However, the long-lived plasma cell is likely the most important in regards to long term antibody production and also the most difficult to eliminate. These non-dividing cells can survive for decades in special niches in the spleen or bone marrow maintained by a delicate balance of cytokines and adhesion signals [33, 34].
Regardless of the efficacy of bortezomib in treating AMR, targeting the plasma cell remains a focus for those interested in developing therapies for both acute and chronic AMR. Other proteasome inhibitors have become available, and new therapies aimed to interrupt the survival signals needed for the survival of the long-lived plasma cell are being developed.
Eculizumab
In the early 1990s, C4d staining of renal tissue became reliable and increasingly utilized. An inactive split product resulting from the cleavage of C4b; C4d covalently binds to the site of complement activation, which is usually vascular endothelium in the renal allograft. With advances in tissue typing techniques, an association between donor specific antibody and C4d staining was made. Subsequently, several clinical studies have shown that positive peritubular capillary staining is not only associated with antibody mediated damage but poor clinical outcomes. Although C4d negative cases of acute antibody mediated rejection exist [35], complement appeared to play a role in antibody mediated damage. A potential C3 inhibitor, Yunnan-cobra venom factor (Y-CVF), has been shown to prevent acute antibody mediated damage in primates [36]. Eculizumab, a humanized monoclonal antibody against C5 that ultimately blocks the membrane attack complex has been studied in humans [3].
Our group treated 26 highly sensitized positive crossmatch kidney transplant recipients with eculizumab at the time of transplant, weekly for the first 4 weeks, and monthly up to 1 year post transplant in patients with persistently high donor specific antibody levels. We found a dramatic reduction in acute antibody mediated rejection. The rate of antibody mediated rejection as defined by high DSA, C4D positivity, and acute allograft dysfunction, only developed in 7.7% of patients as compared to 41.2% of historical controls[3]. The protocol biopsy specimens with C4d positivity were in patients were high donor specific antibody levels typically associated with acute allograft dysfunction. A complement independent mechanism of acute antibody mediated rejection likely exists given that 2 patients developed acute AMR despite complement blockade. However, the results of this trial strongly suggest that complement plays a major role in mediating antibody damage in the transplanted kidney. A randomized multicenter open-label trial of eculizumab versus standard therapy is currently being conducted and results are unavailable. The effect of eculizumab on chronic antibody mediated rejection is largely unknown at this time.
Unfortunately the efficacy of the available treatments for antibody mediated rejection is variable. The differences in patient populations and study design accounts for some of the variability in outcome, but there are differences in whether the patient has pre-formed antibody or antibody develop soon after transplant. In the highly sensitized positive crossmatch population, long-lived terminally differentiated plasma cells that reside in the bone marrow and other secondary lymphoid tissue are difficult to target and antibody is continually produced. In other situations antibody production is the result of memory B-cell stimulation and rapid conversion to plasma cells.
Conclusions
The ability to diagnose and treat acute AMR is a necessary skill for clinicians caring for renal transplant recipients today. Most cases of acute AMR can be reversed if treated promptly and aggressively. While we have outlined our programmatic approach, we also recognize that other therapies might be equally or even more effective and have tried to present these approaches here. We contend that while acute AMR is manageable, the major unmet need with respect to DSA is the prevention of chronic injury. A better understanding of the biology of chronic AMR and new therapeutic modalities will be need to approach this difficult problem
Abbreviations
- AMR
acute antibody mediated rejection
- DSA
donor specific antibody
- MFI
mean fluorescence intensity
- PE
plasmapheresis
- IVIG
intravenous immunoglobulin
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Carrie Schinstock declares that she has no conflict of interest.
Mark D. Stegall reports grants from Alexion, grants from Millennium, and personal fees from iPerion.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Recent papers of particular interest have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Gloor JM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):582–9. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 2.Burns JM, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(12):2684–94. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 3•.Stegall MD, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(11):2405–13. doi: 10.1111/j.1600-6143.2011.03757.x. This study is important because it is the first large clinical series in using eculizumab to show dramatic reduction in acute antibody mediated rejection in positive crossmatch kidney transplant recipients. [DOI] [PubMed] [Google Scholar]
- 4•.Dorje C, et al. Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation. 2013;96(1):79–84. doi: 10.1097/TP.0b013e31829434d4. Important study of the difference in outcomes between patients with early versus late acute antibody mediated rejection. [DOI] [PubMed] [Google Scholar]
- 5.Walsh RC, et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91(11):1218–26. doi: 10.1097/TP.0b013e318218e901. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Reed EF. Effect of antibodies on endothelium. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(11):2459–65. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CY, et al. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84(10):1324–34. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 8.Gurland HJ, et al. Plasmapheresis in renal transplantation. Kidney international. Supplement. 1983;(14):S-82–4. [PubMed] [Google Scholar]
- 9.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation. 2012;94(8):775–83. doi: 10.1097/TP.0b013e31825d1587. [DOI] [PubMed] [Google Scholar]
- 10.Raghavaiah S, Stegall MD. New therapeutic approaches to antibody-mediated rejection in renal transplantation. Clinical pharmacology and therapeutics. 2011;90(2):310–5. doi: 10.1038/clpt.2011.123. [DOI] [PubMed] [Google Scholar]
- 11.Jordan SC, et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation. 1998;66(6):800–5. doi: 10.1097/00007890-199809270-00017. [DOI] [PubMed] [Google Scholar]
- 12.Jordan SC, et al. Current approaches to treatment of antibody-mediated rejection. Pediatric transplantation. 2005;9(3):408–15. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 13.Casadei DH, et al. A randomized and prospective study comparing treatment with high-dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid-resistant rejection. Transplantation. 2001;71(1):53–8. doi: 10.1097/00007890-200101150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Luke PP, et al. Reversal of steroid- and anti-lymphocyte antibody-resistant rejection using intravenous immunoglobulin (IVIG) in renal transplant recipients. Transplantation. 2001;72(3):419–22. doi: 10.1097/00007890-200108150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Vo AA, et al. Safety and adverse events profiles of intravenous gammaglobulin products used for immunomodulation: a single-center experience. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(4):844–52. doi: 10.2215/CJN.01701105. [DOI] [PubMed] [Google Scholar]
- 16.Kahwaji J, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(12):1993–7. doi: 10.2215/CJN.04540709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke JE, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(4):842–6. doi: 10.1111/j.1600-6143.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 18.Tzvetanov I, et al. The role of splenectomy in the setting of refractory humoral rejection after kidney transplantation. Transplantation proceedings. 2012;44(5):1254–8. doi: 10.1016/j.transproceed.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan B, et al. Histopathology and immunophenotype of the spleen during acute antibody-mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(5):1316–20. doi: 10.1111/j.1600-6143.2010.03067.x. [DOI] [PubMed] [Google Scholar]
- 20.Tzvetanov I, et al. Cell population in spleens during antibody-mediated rejection: pathologic and clinical findings. Transplantation. 2012;94(3):255–62. doi: 10.1097/TP.0b013e3182562881. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga M, et al. Down-regulation of CD40 and CD80 on B cells in patients with life-threatening systemic lupus erythematosus after successful treatment with rituximab. Rheumatology. 2005;44(2):176–82. doi: 10.1093/rheumatology/keh443. [DOI] [PubMed] [Google Scholar]
- 22.Sanz I, et al. Phenotypic and functional heterogeneity of human memory B cells. Seminars in immunology. 2008;20(1):67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister S, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer research. 2007;67(4):1783–92. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 24.Perry DK, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(1):201–9. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 25.Diwan TS, et al. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011;91(5):536–41. doi: 10.1097/TP.0b013e3182081333. [DOI] [PubMed] [Google Scholar]
- 26.Everly MJ, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86(12):1754–61. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 27.Walsh RC, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89(3):277–84. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 28.Wong W, et al. Bortezomib in kidney transplant recipients with antibody mediated rejection: three case reports. Clinical transplants. 2009:401–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Sberro-Soussan R, et al. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: 4 case reports. Clinical transplants. 2009:433–8. [PubMed] [Google Scholar]
- 30.Sberro-Soussan R, et al. Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):681–6. doi: 10.1111/j.1600-6143.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 31.Sberro-Soussan R, et al. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: even after one year post-treatment. Clinical transplants. 2010:409–14. [PubMed] [Google Scholar]
- 32.Schmidt N, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation. 2012;94(4):352–61. doi: 10.1097/TP.0b013e318257acf6. [DOI] [PubMed] [Google Scholar]
- 33.Hoyer BF, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. The Journal of experimental medicine. 2004;199(11):1577–84. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassese G, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. Journal of immunology. 2003;171(4):1684–90. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 35.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Current opinion in organ transplantation. 2010;15(1):42–8. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 36.Chen Song S, et al. Complement inhibition enables renal allograft accommodation and longterm engraftment in presensitized nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2057–66. doi: 10.1111/j.1600-6143.2011.03646.x. [DOI] [PubMed] [Google Scholar]
- 37.Bonomini V, et al. Effects of plasmapheresis in renal transplant rejection. A controlled study. Transactions - American Society for Artificial Internal Organs. 1985;31:698–703. [PubMed] [Google Scholar]
- 38.Kirubakaran MG, et al. A controlled trial of plasmapheresis in the treatment of renal allograft rejection. Transplantation. 1981;32(2):164–5. doi: 10.1097/00007890-198108000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Allen NH, et al. Plasma exchange in acute renal allograft rejection. A controlled trial. Transplantation. 1983;35(5):425–8. doi: 10.1097/00007890-198305000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Blake P, Sutton D, Cardella CJ. Plasma exchange in acute renal transplant rejection. Progress in clinical and biological research. 1990;337:249–52. [PubMed] [Google Scholar]
- 41.Stegall MD, et al. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(2):346–51. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 42.Glotz D, et al. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993;56(2):335–7. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Tyan DB, et al. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation. 1994;57(4):553–62. [PubMed] [Google Scholar]
- 44.Lefaucheur C, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(5):1099–107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaposztas Z, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clinical transplantation. 2009;23(1):63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]