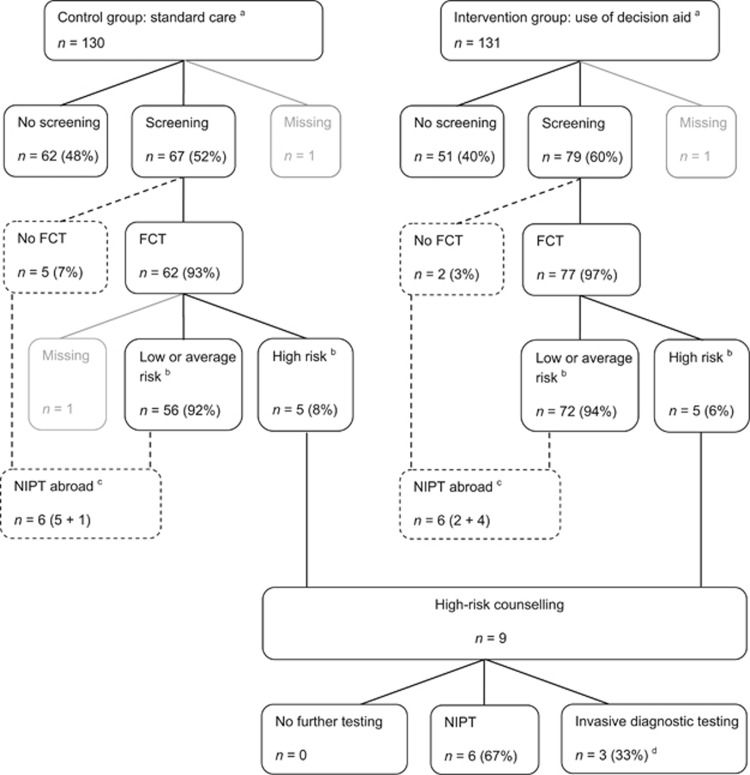
Figure 2.
Prenatal test utilisation of study participants. Dashed lines indicate pathways outside the current programme for prenatal screening and diagnosis in the Netherlands. aPrenatal test utilisation based on self-report by participants during the process of decision making (prenatal test intended, n=5) or when the decisional process was completed and, where applicable, prenatal testing for chromosomal abnormalities was performed and the outcome was known (prenatal test confirmed, n=254). bCut-off risk FCT 1: ≤200. cOffering NIPT as primary screening test is currently prohibited in The Netherlands, but available in neighbouring countries. dTwo participants had invasive diagnostic testing performed after FCT had revealed a nuchal translucency ≥3.5 mm. One participant had invasive diagnostic testing performed after FCT had shown maternal serum markers (PAPP-A and free βhCG) to be suggestive for fetal triploidy. FCT, first-trimester combined test; NIPT, non-invasive prenatal test.