Figure 3.
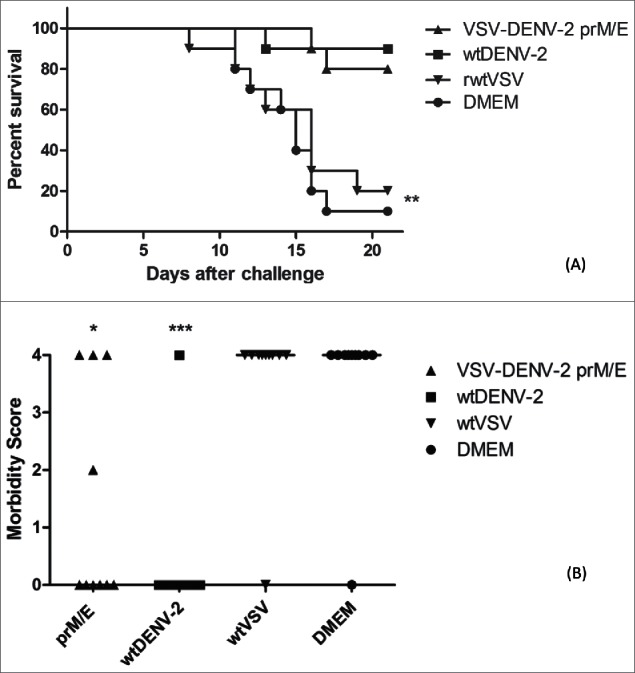
Mouse protection experiments against challenge with 50LD50 of DENV-2. Groups of ten animals were immunized with 1 × 106 PFU of DENV-2 TR1751/animal, VSV-DENV-2 vaccine candidate, recombinant wild type VSV vector or only DMEM, in a two doses schedule 15 d apart, and challenged two weeks after the second inoculation. Mortality (A) and morbidity (B) were observed for 21 d. 80% and 90% of animals immunized with VSV-DENV-2 candidate vaccine and the control DENV-2 respectively are protected after lethal challenge. Two of surviving animals in the VSV-DENV-2 group had mild and severe paralysis, but the morbidity were statistically different of DMEM or wtVSV group. Asterisks indicate statistically significant differences between vaccinated animals and mice inoculated with the control rwtVSV or DMEM (*p < 0 .05 and ***p < 0 .001). Data represent one of three independent experiments with 10 animals per group (n = 40).
