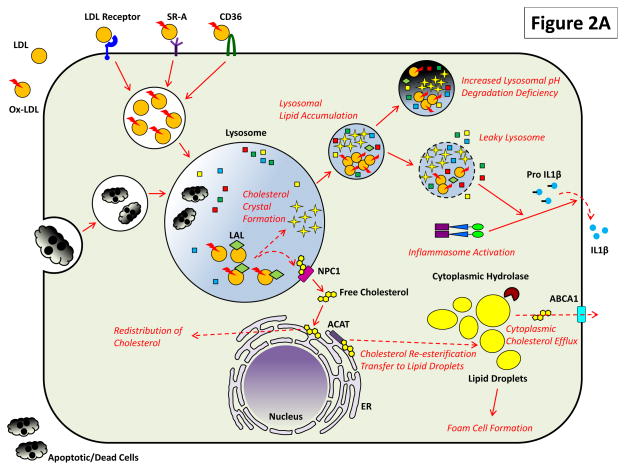
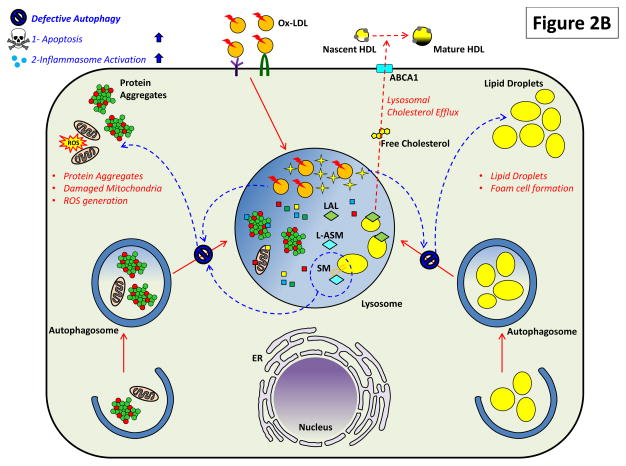
Figure 2. The Critical Roles of Macrophage Lysosomes in Atherosclerosis.
Both (A) heterophagy and (B) autophagy play important roles in the atherosclerotic macrophage and impairment of these pathways has deleterious effects.
(A) LDL, especially upon its modification/oxidation and detection by scavenger receptors such as SR-A and CD36, is endocytosed to lysosomes for hydrolysis and redistribution. Cholesteryl esters are acted on by lysosomal acid lipase (LAL) to generate free cholesterol which is transferred to ER via a mechanism requiring lysosomal proteins NPC2 and NPC1. From there, free cholesterol is either distributed to other membrane compartments or is re-esterification by ACAT on ER and transferred to lipid droplets. Excess cholesterol accumulation is balanced by the action of cytoplasmic hydrolases on lipid droplets upon which cholesterol is removed from the cell via an ABCA1-dependent cholesterol efflux. Lipid overload in atherosclerosis directly affects lysosomal degradation: modified LDL and cholesterol crystals – a byproduct of hydrolyzed but poorly handled cholesterol - accumulates in lysosomes and render them dysfunctional by preventing optimal acidification and inducing lysosomal leakage. Proatherogenic consequences of this dysfunction include activation of the inflammasome/secretion of IL-1β and an inability to clear apoptotic/dead cells by phagocytosis (efferocytosis).
(B) Lysosomal lipid accumulation/lysosomal dysfunction also prevents optimal autophagic degradation (shown by blue arrows). This could be mediated by similar mechanisms affecting heterophagic degradation as well as others such as accumulation of sphingomyelin. Reduction in autophagy also has several deleterious effects on atherosclerosis. Macrophages lose a parallel mechanism of cellular cholesterol removal mediated by autophagic engulfment of lipid droplets (lipophagy), hydrolysis by LAL, and efflux of cholesterol again by an ABCA1-dependent process. Blockage of this alternate route for cholesterol efflux induces lipid droplet accumulation and foam cell formation. Deficient autophagy also affects homeostatic turnover of organelles (like mitochondria) and the clearance of protein aggregates. Although accumulation of protein aggregates and dysfunctional organelles have not been directly studied in atherosclerosis, their ability to trigger deleterious effects such as ROS generation, apoptosis, and inflammasome activation can be surmised to exacerbate plaque progression.