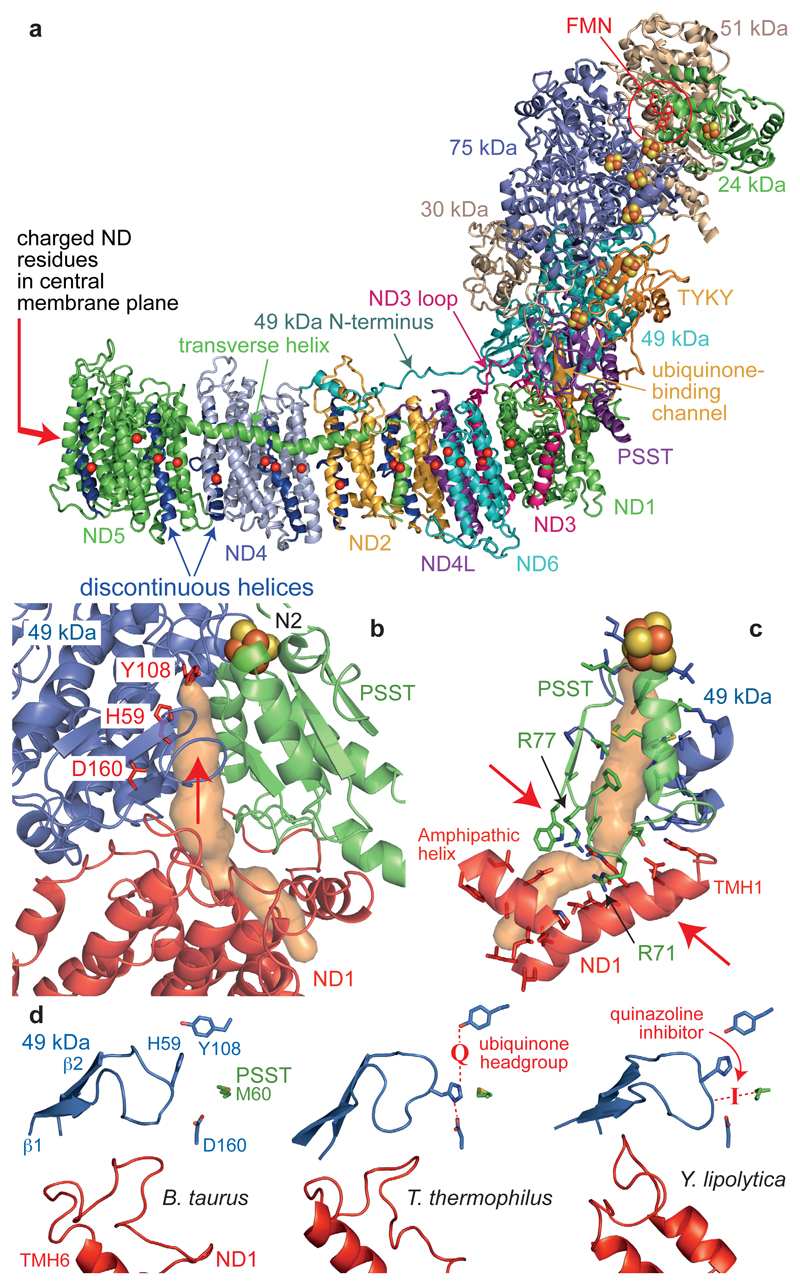
Figure 3. The core subunits and ubiquinone-binding site of mammalian complex I.
a) The core subunits with the FMN, FeS clusters, conserved charged residues (Cα) in the membrane (overlaid for clarity), and proposed ubiquinone-binding channel (orange). b) The ubiquinone-binding channel. 49 kDa-Tyr108 and 49 kDa-His599 form hydrogen bonds to the bound ubiquinone at the top of the cleft (indicated by an arrow) between 49 kDa and PSST. The channel entrance is between three helices in ND1. c) Structural elements forming the channel and bottleneck (between the arrows). PSST-Arg77 is hydroxylated23. d) Conformations of the 49 kDa (β1-β2) and ND1 (TMH5-6) loops observed in different species. In T. thermophilus9 the ubiquinone headgroup binds between Tyr108 and His59 and His59 hydrogen bonds to 49 kDa-Asp160. In Y. lipolytica11 a quinazoline inhibitor is bound between PSST-Met60 and the tip of the 49 kDa loop.