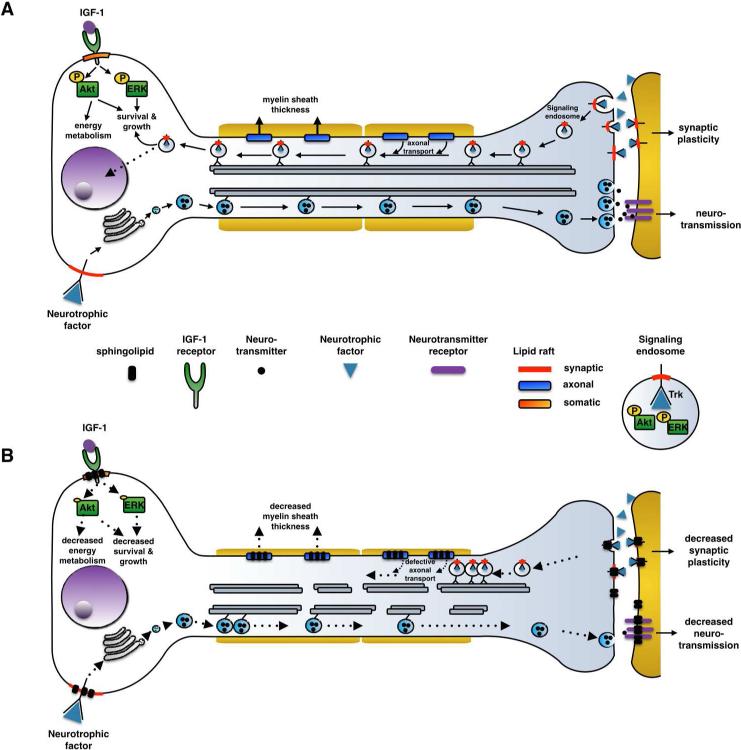
Figure 1.
Model of dysregulation of neuronal survival pathways in sphingolipidoses. A) In a healthy neuron somatic, axonal and synaptic membranes contain lipid rafts - possibly with differing compositions- that contribute to distinct survival functions based on regional signaling needs. In the synapse, target-derived neurotrophic factors activate raft associated Trk receptors on both the pre- and post-synaptic side. Ligand-bound Trk activates pathways important for synaptic plasticity in the post-synaptic neuron/dendrite. On the pre-synaptic side, Trk receptors are internalized by endocytosis and form ‘signaling endosomes’ complete with activated downstream effectors such as phospho-Akt and phospho-Erk. Signaling endosomes are then transferred to the cell body through retrograde axonal transport along microtubules, where they trigger a survival response. Axonal lipid rafts may contribute to this process by coordinating signaling molecules that regulate the functions of dynein and kinesin motors. In a separate role, axonal rafts could also mediate signals that regulate myelin sheath thickness around the axon. In contrast, somatic rafts mediate IGF-1 and neurotrophic receptor signaling that are important for cellular energy metabolism needed for growth and survival, and secretion of neurotransmitters respectively. B) In sphingolipidoses, the accumulation of undigested lipids in rafts disrupts signaling pathways important for the function of that particular membrane region. There could be regional differences in the extent of signaling activity that depends on intact lipid rafts, and could explain the differential sensitivity observed especially in the synapse, which is first to fail in function. Decreased receptor internalization at the synapse due to changes in biophysical characteristics of the membrane followed by microtubule destabilization and axonal transport defects lead to dying-back pathology, and eventually neuronal apoptosis.