Abstract
An ideal malaria vaccine should target several stages of the parasite life cycle and induce anti-parasite and anti-disease immunity. We have reported a Plasmodium yoelii chimeric multi-stage recombinant protein (PyLPC/RMC), engineered to express several autologous T cell epitopes and sequences derived from the circumsporozoite protein (CSP) and the merozoite surface protein 1 (MSP-1). This chimeric protein elicits protective immunity, mediated by CD4+ T cells and neutralizing antibodies. However, experimental evidence from pre-erythrocytic vaccine candidates and irradiated sporozoites has shown that CD8+ T cells play a significant role in protection. Recombinant viral vectors have been used as a vaccine platform to elicit effective CD8+ T cell responses. The human adenovirus serotype 5 (Ad5) has been tested in malaria vaccine clinical trials with excellent safety profile. Nevertheless, a major concern for the use of Ad5 is the high prevalence of anti-vector neutralizing antibodies in humans, hampering its immunogenicity. To minimize the impact of anti-vector pre-existing immunity we developed a chimeric Ad5/3 vector in which the knob region of Ad5 was replaced with that of Ad3, conferring partial resistance to anti-Ad5 neutralizing antibodies. Furthermore, we implemented heterologous adenovirus/protein immunization regimens which include a single immunization with recombinant Ad vectors. Our data show that immunization with the recombinant Ad5/3 vector induces protective efficacy indistinguishable from that elicited by Ad5. Our study also demonstrate that the dose of the Ad vectors has an impact on the memory profile and protective efficacy. The results support further studies with Ad5/3 for malaria vaccine development.
Introduction
Malaria continues to be the most relevant parasitic disease. Although significant improvements in malaria control have occurred in the past few years, the number of clinical episodes worldwide has been estimated at 198 million with 584,000 deaths annually (1). An effective vaccine is required to reduce the burden of the infection and ultimately reduce transmission. A majority of the malaria vaccines that have reached clinical trials have been focused on targeting single antigens. However, given the complexity of the parasite-host interaction, an ideal malaria vaccine should target several stages of the parasite life cycle to induce clinical and anti-disease immunity. Although both antibody-mediated and cellular effector mechanisms have been considered critical for anti-malaria immunity, a formulation able to induce such balanced immune responses is not yet available.
We have previously reported P. yoelii chimeric recombinant proteins that are able to elicit protective immunity using stringent murine challenge models (2, 3). These proteins include sequences derived from the circumsporozoite protein (CSP) a pre-erythrocytic stage antigen that we have called Linear Peptide Chimera (LPC) and the merozoite surface protein 1 (MSP-1) that we have called Recombinant Modular Chimera (RMC). Experiments have shown that these chimeric proteins, engineered to contain several autologous promiscuous T cell epitopes, have superior efficacy compared to a non-chimeric vaccine constructs (2). The potential synergistic effect of combining these novel antigens was subsequently investigated by comparing the immune responses after administration of the proteins formulated as a mixture or delivered as a single fusion protein (P. yoelii LPC/RMC [PyLPC/RMC]) (4). We confirmed that both approaches were effective in inducing multi-stage immune responses.
In vivo depletion of CD4 and/or CD8 T cells, in addition to passive transfer experiments of purified total IgG, showed that protective immunity induced by immunization with PyLPC/RMC was mediated by CD4+ T cells and neutralizing antibodies (4). Experimental evidence using a diverse set of pre-erythrocytic stage vaccine candidates or irradiated sporozoites have shown that CD8+ T cells also play a significant role in protection against the sporozoite challenge by interfering with liver stage development. In the search of optimal vaccine platforms, several recombinant viral vectors have been used to deliver pre-erythrocytic vaccine candidates designed to elicit effective CD8+ T cell responses (5). On the basis of such observations, we decided to produce recombinant adenovirus vectors expressing PyLPC/RMC as a transgene and tested several prime-boost immunization regimens with the reported fusion protein in an effort to improve protective efficacy.
Adenovirus vectors were selected due to the broad safety profile and ability to induce a robust CD8+ T cells and antibody responses (5). The most broadly used adenovirus vector is the human adenovirus serotype 5 (Ad5). However, a major concern for the use of Ad5 is that the high prevalence of anti-vector neutralizing antibodies in humans hampering its immunogenic potential (6). Two different strategies are reported here to reduce the effect of anti-vector pre-existing immunity: implementation of a single immunization scheme with recombinant Ad vectors using heterologous prime-boost immunization regimens and the use of the chimeric Ad5/3 vector that is able to circumvent anti-Ad5 preexisting immunity (7). The knob region of the fiber protein has been described as the main domain where the immune responses are directed after a natural infection (8). The Ad5/3 vector has the Ad5 knob region replaced by that of Ad serotype 3 (Ad3) knob. We evaluated the magnitude and functional properties of the immune responses elicited by immunization with recombinant Ad5 and recombinant Ad5/3 expressing the chimeric PyLPC/RMC antigen as a transgene and tested several immunization regimens. Comparative experiments demonstrated the immune responses elicited by immunization with recombinant Ad5 or recombinant Ad5/3 were similar. Most relevantly, our data show that the Ad5/3 immunogenicity is related with protection, supporting the use of Ad5 capsid modified vectors for malaria vaccine development.
Materials and Methods
Chimeric vaccines design and characterization
The synthetic genes encoding for the P. yoelii pre-erythrocytic/erythrocytic stage antigens have been previously described (4). A 1242 bp synthetic Py-lpc/rmc gene, encoding a chimeric antigen based on the P. yoelii circumsporozoite protein genetically linked to a chimeric P. yoelii MSP-1 was transformed into E. coli BL21 (DE3) cells (Novagen, Madison, WI), and protein expression induced with 1 mM IPTG for 3 hours. The 414 amino acid hybrid protein was purified with a Ni-NTA affinity column according to the manufacturer’s instructions (Qiagen, Valencia, CA). For adenovirus expression, the gene was codon-optimized for mammalian expression and synthesized commercially by Geneart (Regensburg, Germany). The Py-lpc/rmc gene was cloned into the shuttle vector pAdTrackCMV. The resulting shuttle plasmid pCMVLPC/RMC was co-transformed with the Ad backbone E1/E3-negative plasmids, pAdEasy-1 or with pAd5/3Easy (7), into the E. coli strain, BJ5183. The BJ5183 strain is recA proficient and supplies the machinery necessary for efficient homologous recombination between the shuttle plasmid and the Ad backbone plasmid. After selection on kanamycin, recombinants were screened by restriction digestion and PCR analyses for the presence of the Pylpc/rmc gene insert. Positive plasmids were then transformed into a second E. coli strain, XL10-Gold (Stratagene), for amplification of the recombined Ad plasmids. The recombinant Ad plasmids were then transfected into HEK293 cells. HEK293 cells complement the E1 deletion in the Ad backbone plasmid and allow de novo production of Ad virions. Recombinant Ad vectors were rescued, expanded and purified by double cesium chloride sedimentation centrifugation. The physical titers, or total virus particles (VP), were determined spectrophotometrically by measuring the O.D. at 260 nm. Infectious titers were determined on HEK293 cells using a 50% end-point dilution (TCID50) assay. The expression of PyLPC/RMC was confirmed by western blot and flow cytometry analysis as described (9).
Virus neutralization assays
To assess the ability of anti-Ad5 antibodies to neutralize the chimeric Ad5/3 vector, we generated anti-vector antibodies in mice. For these experiments groups of CB6F1/J mice received two immunizations with 1.4x107 vp of empty Ad5 or empty Ad5/3 vectors twenty days apart and the sera tested for neutralization activity against Ad5 and Ad5/3 vectors expressing luciferase as described (10). Neutralization titers were defined as the maximum serum dilution that neutralized 50% of luciferase activity.
Peptides
A library of 58 synthetic peptides representing the complete amino acid sequence of the hybrid protein was used for ex vivo stimulation. The 15-mer synthetic peptides were overlapped by 10 amino acids and commercially synthesized by Sigma-Aldrich (St Louis, MO). The peptides were dissolved in dimethyl sulfoxide and stock solutions stored at −20°C. For ex vivo stimulation three peptide pools were used: the first pool contained 14 peptides that represented the PyLPC sequence (PyLPC pool), the second pool contained 24 peptides that represented the region of the PyRMC chimeric protein that contains T cell epitopes (PyRMC pool 1), and the third pool contained 20 peptides that represented the PyMSP-119 sequence (PyRMC pool 2) (4). The H-2Kd/SYVPSAEQI/APC tetramer was synthesized at the Tetramer Core Facility (Emory University, Atlanta, GA) representing the CTL epitope of the P. yoelii CSP included in the chimeric LPC protein.
Immunization regimens and parasites
Female CB6F1/J (H-2d/b) mice, 6 to 8 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). The hybrid mice were selected based on our published data concerning the response of syngeneic mice to chimeric antigens (11) and to characterize SYVPSAEQI-specific CD8+ T cells (H-2Kd restricted). Mice were housed in micro-isolation cages and all procedures were approved by Emory University’s Institutional Animal Care and Use Committee and followed accordingly. The mice were immunized subcutaneously for protein immunization or intramuscularly for adenovectored vaccines on days 0, 20 and 40 as summarized in Table I. The chimeric protein was used at 20 μg emulsified in Montanide ISA 51 (Seppic, Fairfield, N.J.) and recombinant adenovirus vectors adjusted at 1.4x107 or 1x1010 vp/dose. The following groups were tested (Table I): 1) Homologous prime-boost with Ad5-Pylpc/rmc. 2) Homologous prime-boost with Ad5/3-Pylpc/rmc.3) Homologous prime-boost with the PyLPC/RMC recombinant protein. 4) Two immunizations with the recombinant protein and a final boosting with Ad5-Pylpc/rmc. 5) Two immunizations with the recombinant protein and boosting with Ad5/3-Pylpc/rmc. 6) Priming with Ad5-Pylpc/rmc and boosting twice with the recombinant protein. 7) Priming with Ad5/3-Pylpc/rmc and boosting twice with the recombinant protein. 8) Priming and boosting with Ad5-Pylpc/rmc. 9) Priming and boosting with Ad5/3-Pylpc/rmc. 10) Control group that received priming and boosting with an Ad5 empty vector. 11) Control group that received priming and boosting with an Ad5/3 empty vector. For Ad5 sensitization, female CB6F1/J (H-2d/b) mice, 6 to 8 weeks of age, were immunized intramuscular with empty Ad5 vectors at a dose of 107 vp at days 0 and 20 to generate neutralizing anti Ad5 antibodies, 20 days after the sensitization mice received a priming with 107 v.p. of Ad5 or Ad5/3 expressing PyLPC/RMC as a transgene followed by two boosting immunizations with PyLPC/RMC at 20 days’ intervals.
Table I.
Immunization Regimens
| Group | Priming (Day 0) | Boosting (Day 20) | Second Boosting (Day 40) |
|---|---|---|---|
| Homologous Immunizations | |||
| Ad5 | Ad5-Pylpc/rmc 1 | Ad5-Pylpc/rmc1 | No Immunization |
| Ad5/3 | Ad5/3-Pylpc/rmc 1 | Ad5/3-Pylpc/rmc1 | No Immunization |
| Prime-Boost Regimes | |||
| PPP | PyLPC/RMC | PyLPC/RMC | PyLPC/RMC |
| PPAd5 | PyLPC/RMC | PyLPC/RMC | Ad5-Pylpc/rmc1 |
| PPAd5/3 | PyLPC/RMC | PyLPC/RMC | Ad5/3-Pylpc/rmc1 |
| Ad5PP | Ad5-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Ad5/3PP | Ad5/3-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Regimens in Ad5 Sensitized mice2 | |||
| Ad5e-Ad5PP | Ad5-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Ad5e-Ad5/3PP | Ad5/3-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Dose Comparison Regimens | |||
| Ad5-S | Ad5-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Ad5-H | Ad5-Pylpc/rmc3 | PyLPC/RMC | PyLPC/RMC |
| Ad5/3-S | Ad5/3-Pylpc/rmc1 | PyLPC/RMC | PyLPC/RMC |
| Ad5/3-H | Ad5/3-Pylpc/rmc3 | PyLPC/RMC | PyLPC/RMC |
| Control Groups | |||
| Ad5e | Ad54 | Ad54 | No Immunization |
| Ad5/3e | Ad5/34 | Ad5/34 | No Immunization |
| Ad5e-M | Ad54 | Montanide ISA51 | Montanide ISA51 |
| Ad5/3e-M | Ad5/34 | Montanide ISA51 | Montanide ISA51 |
Recombinant Ad vector dose 1x107 vp.
Previous to the immunizations, mice received two immunizations with Ad5 107 vp. without a transgene 20 days apart to induce Ad5 neutralizing ab
Recombinant Ad vector dose 1x1010 vp.
No Transgene. Ad vector dose 1x107 vp.
Anopheles stephensi P. yoelii 17XNL infected mosquitoes were obtained from the New York University School of Medicine insectary core facility. Experimental challenges were done intravenously using 100 freshly isolated sporozoites. Giemsa-stained thin smears were made daily and used to quantify parasitemia by counting the percentage of infected erythrocytes from at least 1000 counted cells per mouse using light microscopy. Additional blood samples were used to determine hemoglobin concentration as described using a Hemocue Hb 201+ hemoglobinometer (HemoCue, Brea, CA) (4). Mice were classified as severely anemic when hemoglobin levels reached 5 g/dL. Those animals with a hemoglobin concentration lower than this cutoff value were euthanized.
ELISA assays
The antibodies elicited by immunization with the hybrid protein were determined by ELISA using Immulon 4HB plates (Thermo Labs Systems, Franklin, MA) coated with 1 μg/ml of the hybrid protein diluted in carbonate buffer as described (2). Optical densities were determined using a VERSAmax ELISA reader (Molecular Device Corporation, Sunnyvale, CA) with a 405 nm filter. The endpoint was measured as the highest dilution of sera that resulted in an O.D. of 0.5 (OD0.5). The results are presented as the reciprocal of the end-point dilution. IgG isotype profiles were also determined by ELISA as described (2). The affinity of antibodies was assessed by a thiocyanate elution-based ELISA as described previously (12).
T cell depletion experiments
Depletion of lymphocyte subsets were conducted with groups of four or five mice by intravenous inoculation of purified rat anti-CD4 (clone GK1.5) and anti-CD8 (clone YTS169.4) monoclonal antibodies. Control mice, immunized with the specified vaccination regimen, received purified normal purified rat IgG. As a positive control for infection groups of mice were immunized with the empty Ad5/3 vector followed by two boosting immunizations with Montanide 51 alone. Mice received 500 μg of the specified purified antibodies/mouse given on days −1 and +2, relative to the day of challenge. The efficiency of T cell depletion was assessed by flow cytometry analysis of splenocytes on day five using naïve mice.
Flow cytometry assays
For flow cytometric analysis of the P. yoelii LPC/RMC-specific CD8+ and CD4+ T cells, peripheral blood was collected into 3.7% sodium citrate/PBS tubes and erythrocytes were lysed with ACK buffer (Life Technologies, Carlsbad, CA). After washing, the cells were incubated with α-CD3ε-PerCP (Clone: 145-2C11 Biolegend), α-CD4-Alexa Fluor 700 (Clone: RM4-5 eBioscience), α-CD11a-PerCP-Cy5.5 (Clone: M17/4 Biolegend), α-CD49d-FITC (Clone: R1–2 Biolegend), α-CD8-APC-Cy7 (Clone: 53–6.7 Biolegend), H-2Kd/SYVPSAEQI/APC tetramer (Tetramer Core Facility, Emory University, Atlanta, GA), α-CD62L-V450 (Clone: MEL-14 BD Biosciences), and α-CD127-PE Cy7 (Clone: A7R34 Biolegend) for 1 h at 4°C and then analyzed by flow cytometry. The cells were initially gated on SSC/FSC, and then the frequency of tetramer-positive cells was determined on the gated CD11α+CD8+ population. As a surrogate marker of antigen induced stimulation CD4+ T cells were gated on CD11+ CD49dHi population as previously described (13).
Cellular immune responses in the spleen were measured by ICS, the panel was used to simultaneously analyze IL-2, IFN-γ and TNF-α at the single-cell level in T cells derived from splenocytes obtained 5, 11 or 19 days after boosting. Cells were stimulated for 6 hours with peptide pools, or with PyLPC/RMC at 2 μg/ml, at 37°C in the presence of GolgiPlug (BD Biosciences). Cells were then incubated for 15 min in the presence of anti-mouse CD16/CD32 (Fc-block) before surface staining for 30 min with α-CD3ε-PerCP-Cy5.5 (145-2C11 Biolegend), α-CD8α-BV605 (Clone: 53–6.7 Biolegend), and α-CD4-Pacific Blue (Clone: GK1.5 Biolegend). Permeabilization was performed using Cytofix/Cytoperm solution (BD Biosciences) according to the manufacturer’s instructions. Cells were stained intracellularly for 30 min with α-IFN-γ-FITC (Clone: XMG1.2 Biolegend), α-IL-2-APC (JES6-5H4 Biolegend), and α-TNF-α-PE (Clone: TN3-19.12 Biolegend). All incubations were performed at 4°C. Cells were resuspended in 1% PFA solution and Flow cytometry analyses were performed using a LSRII (BD Biosciences, San Jose, CA), data were analyzed using FlowJo V10 (Tree Star, Ashland, OR). Analyses of multifunctional T cell responses were done using a Boolean analysis in FlowJo. The lymphocytes were initially gated on CD3+CD4+ and CD3+CD8+, then antigen-specific cytokine-secreting T cells were identified. The frequency of antigen-specific cytokine-producing cells was determined by subtracting the percentage of cytokine producing T cells after incubation with medium alone from the percentage of cytokine-producing T cells after incubation with PyLPC/RMC, or the corresponding peptide pools. A threshold for a positive cytokine response was set above the background, and samples that did not meet this requirement were set to zero.
Statistics
Statistical analysis and graphs were made using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA). For analysis of the homologous vaccination regimens antibody responses and tetramer staining data were log-transformed to achieve normality, permitting parametric testing and comparisons using Student’s paired t-test. For heterologous vaccination regimens, antibody responses and tetramer staining data were log-transformed to achieve normality, permitting parametric testing and comparison using one-way ANOVA with Bonferroni’s post-test. For antibody affinity assays and cytokine secreting cells, Kruskal Wallis test with Dunn’s post-test was used. In experimental challenges, differences between groups were evaluated by comparing mean area under the curve (AUC) values using one-way ANOVA with Bonferroni’s post-test.
Results
The high frequency of pre-existing immunity to adenovirus serotype 5 (Ad5) in humans is a major concern for the use of recombinant adenovirus vectors for vaccine development (6). The capsid and fiber modified vectors produced have been demonstrated to reduce the impact of preexisting anti-Ad5 antibodies (14). Here we have tested the fiber-chimeric Ad5/3 vector in which the Ad5 knob region of the fiber has been exchanged with the corresponding region from Ad serotype 3 (Ad3) as previously described (15), for comparative experiments with Ad5. The PyLPC/RMC protein was expressed as a transgene and the recombinant vectors used for immunogenicity and protective efficacy in mice.
Homologous prime-boost immunization regimens with recombinant Ad vectors are highly immunogenic
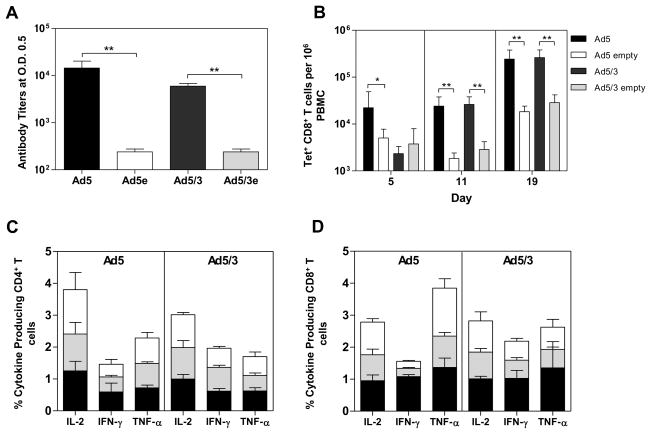
Comparative experiments were done to assess the immunogenicity of recombinant Ad vectors expressing PyLPC/RMC. CB6F1/J mice were immunized twice intramuscularly with 107 vp/dose of Ad5 or Ad5/3 expressing PyLPC/RMC twenty days apart. PyLPC/RMC specific antibody responses were determined 20 days after the boosting immunization by ELISA. Both the unmodified recombinant Ad5 vector and the fiber-modified Ad5/3 induced high antibody titers which ranged between 5.1 x103 and 4.1 x 104 (Figure 1A), with no statistically significant differences. As expected, significant differences in the production of anti-PyLPC/RMC antibodies were observed when both regimens were compared to control mice that received the empty adenovirus vectors (Figure 1A).
Figure 1. Recombinant Ad5/3 exhibits similar immunogenicity than that of recombinant Ad5.
(A) Comparative anti-PyLPC/RMC antibody titers measured 20 days after the second immunization. Endpoint ELISA titers at 0.5 optical density were determined using the recombinant protein PyLPC/RMC and serum samples from mice immunized with recombinant Ad5, recombinant Ad5/3 or empty viral vectors and expressed as arithmetic mean values ± standard deviations (SD). ** p<0.01 by two tailed unpaired Student’s t test. (B) Specific SYVPSAEQI/APC Tet+ CD8+ T cells induced by immunization with Ad5 or Ad5/3 assessed at different time points after the final immunization, expressed as arithmetic mean values ± standard deviations (SD). *p<0.05 **p<0.01 by two tailed unpaired Student’s T test when compared with mice immunized with empty adenoviral vectors. (C) Frequencies of CD4+ or (D) CD8+ T cells producing IL-2, IFN-γ, or TNF-α upon stimulation with pools of synthetic peptides: PyLPC (black bars), PyRMC pool 1 (gray bars), or PyRMC pool 2 (white bars). Bars represent mean responses ± standard deviations for five mice per group.
The frequencies of peptide-specific CD8+ were determined in the peripheral blood at different time points after the last immunization using a combination of antibodies for T cell markers and the H-2Kd/SYVPSAEQI/APC tetramer. A robust CD8+ T cell response was observed regardless of whether the mice were immunized with Ad5 recombinant vector or the fiber-modified Ad5/3 (Figure 1B). Following boosting immunization with the homologous prime-boost immunization regimen, SYVPSAEQI –specific CD8+ T cells were detected 5 days post-immunization. The peak of the SYVPSAEQI-specific CD8+ T cells was observed at day 19. Although differences in the levels of the SYVPSAEQI-specific CD8+ were observed between both adenoviral vectors, with higher numbers observed in the group immunized with Ad5, these differences were not significant by day 11 or day 19 post-immunization (Figure 1B). The breadth of peptide-specific CD4+ and CD8+ T cell responses was assessed by flow cytometry using pools of 15 AA overlapping peptides representing the complete amino acid sequence of the chimeric PyLPC/RMC protein and intracellular cytokine staining. There was comparable production of IL-2, IFN-γ, and TNF-α by both CD4+ and CD8+ T cells in mice immunized with recombinant Ad5 or the fiber-modified Ad5/3 (Figure 1C and D). Cytokine secreting cells were detected after stimulation with peptide pools representing the corresponding LPC or RMC chimeric proteins. There were no statistically significant differences in PyLPC/RMC-specific T cell responses between the two vectors.
Protective efficacy of homologous adenoviral prime-boost regimens
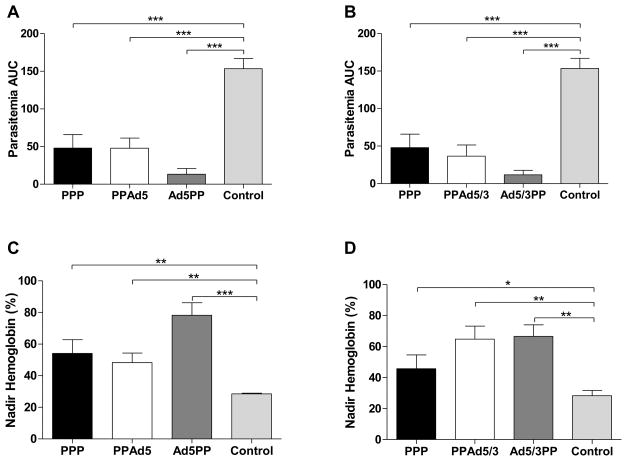
At 30 days after the last immunization, mice were challenged with fresh P. yoelii sporozoites and parasitemias and hemoglobin levels were determined daily. Protective efficacy was assessed using the area under the curve (AUC) of parasitemia vs time and the nadir hemoglobin level, expressed as a percentage of the baseline hemoglobin. These results were compared with a negative control receiving empty Ad5 vectors and a positive control group receiving the previously protective homologous PyLPC/RMC prime-boost immunization regimen (PPP). Of the homologous adenoviral regimens, only the Ad5/3 regimen was able to significantly reduce the parasitemia levels when compared to the control group (Figure 2A). Nonetheless, the Ad5/3 group exhibited a lower protective efficacy than the PPP group. When hemoglobin was evaluated, only the PPP group had an effect on the hemoglobin levels, exhibiting hemoglobin levels twice as high in comparison to the control group at the point when the groups reached the lowest hemoglobin levels (Figure 2B).
Figure 2. Protective effect of homologous Ad5 or Ad5/3 regimens.
Effects of homologous prime-boost regimens based on Ad5 or Ad5/3, after experimental challenge with P. yoelii sporozoites. (A) Parasitemia presented as an area under the curve (AUC) of parasitemia vs time and (B) hemoglobin levels presented as the lowest recorded hemoglobin value expressed as a percentage of the baseline hemoglobin. Results are shown for groups of mice immunized with the specified regimens. Data are the summary of the results of two independent experiments (n=12 mice). Error bars show standard deviations. Immunization groups were compared to a control group receiving empty adenoviral vectors immunizations using one-way ANOVA and Bonferroni’s Post-test *p<0.05, *p<0.0001.
Immunogenicity of heterologous prime-boost vaccination regimens
Considering that the lower efficacy observed in response to the homologous adenoviral regimens could be explained by the reduction in the boosting effect, due to induction of anti-adenoviral antibodies following the adenoviral prime, several heterologous Ad-protein regimens that included only one adenoviral immunization were tested (Table I). To assess the immunogenicity of adenovirus vectors in heterologous prime-boost immunization regimens, we compared antibody and T cell responses in groups of CB6F1/J mice. Recombinant Ad vectors were administered intramuscularly at 107 vp/dose and the PyLPC/RMC chimeric recombinant protein subcutaneously at 20 μg/ml emulsified in Montanide ISA 51. Antibody titers were determined 20 days after the last immunization. High antibody titers ranging between 1.3x106 and 5.2x106 were elicited in the PPP, PPAd5 and PPAd5/3 groups. Significantly lower titers were produced by the Ad priming regimens (ranging between 3.2x105 and 1.3x106) when compared with the homologous prime-boost immunization regimen PPP (p<0.05) (Figure 3A).
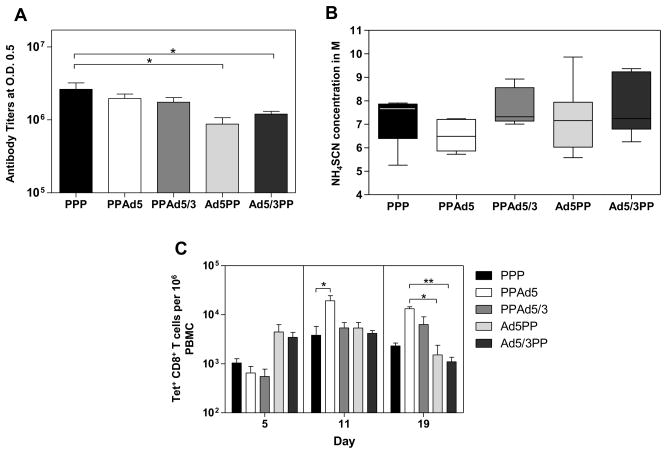
Figure 3. Immunogenicity of prime-boost heterologous regimens.
(A) Anti-PyLPC/RMC antibody titers measured 20 days after the second boosting immunization. Endpoint ELISA titers at 0.5 optical density were determined using the recombinant protein PyLPC/RMC and serum samples from mice immunized with the different heterologous regimens and expressed as arithmetic mean values ± standard deviations (SD). * p<0.05 by one-way ANOVA with Bonferroni’s post-test. (B) Anti-PyLPC/RMC antibody avidity expressed as avidity index measured as the concentration of ammonium thiocyanate required to dissociate 50% of bound antiprotein antibodies. Boxes of box-and-whisker plots summarize the medians and 25th and 75th percentiles, and whiskers indicate the upper and lower adjacent values from 5 mice. (C) Specific SYVPSAEQI/APC Tet+ CD8+ T cells induced by heterologous regimens assessed at different time points after the final immunization, expressed as arithmetic mean values ± standard deviations (SD). * p<0.05 *p<0.01 by Kruskal-Wallis with Dunn’s post-test.
The antibody avidity induced by immunization with several prime-boost immunization regimens was determined using a thiocyanate elution-based ELISA. Although significant differences in final antibody titers were recorded between the mice of different immunization regimens, anti-PyLPC/RMC antibodies exhibited high avidity after the last boosting immunization in all regimens, requiring between 2.7 and 9.8M of NH4NSC to interrupt binding of 50% of the antibodies. There was a trend towards higher avidity in mice immunized with the heterologous regimen that included the recombinant Ad5/3 vector, however these differences were not statistically significant (Figure 3B).
The frequencies of PyLPC/RMC-specific CD8+ were determined in the peripheral blood at different time points after the last boosting immunization using tetramer staining as described above. The mice that received a final boost with recombinant adenovirus boost had significantly higher frequency of SYVPSAEQI-specific T cells (Figure 3C). Following boosting immunization with heterologous prime-boost immunization regimens, SYVPSAEQI –specific CD8+ T cells were also detected 5 days post-immunization with a peak at day 11. Significant differences between groups were found on day 11 between the mice that received the PPAd5 immunization regimen and those immunized with the recombinant protein only (p<0.05). At day 19, the PPAd5 regimen exhibited significantly higher numbers of SYVPSAEQI –specific CD8+ T cells than both adenovirus priming regimens (p<0.05 for Ad5PP and p<0.01 for Ad5/3PP).
Functional quality of the T cell responses induced by the heterologous regimens
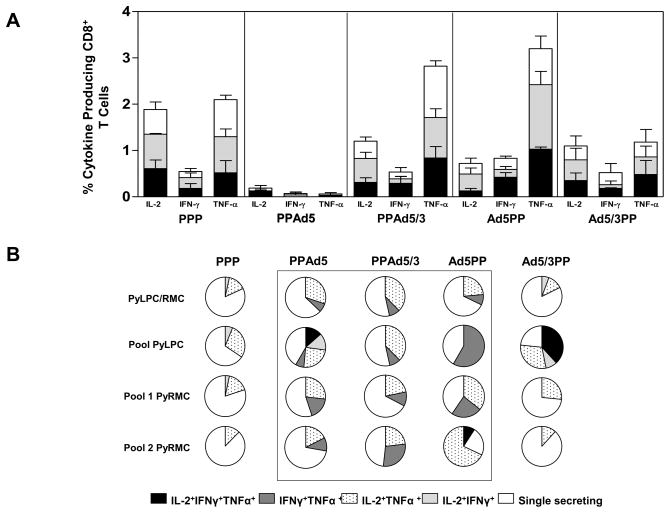
To assess the functional quality of T cells elicited by immunization with several prime-boost immunization regimens, we characterized the frequency of CD4+ and CD8+ T cells able to produce IFN-γ, TNF-α, and IL-2 in response to stimulation with the PyLPC/RMC protein five days after the final boosting immunization. In contrast to our observations following tetramer staining, mice immunized with the homologous prime-boost immunization (PPP) produced high frequencies of CD8+ T cells capable of producing cytokines in response to the three peptide pools when tested five days after the last boosting immunization. These peptide-specific T cells preferentially produced IL-2 and TNF-α (Figure 4A). Additionally, differences in the frequencies of peptide pool specific CD8+ T cells were observed between mice immunized with PPAd5 compared to PPAd5/3, with higher frequencies of cytokine secreting cells induced by PPAd5/3 regimen that included the fiber modified vector (IL-2, p<0.01; IFN-γ, p<0.001 and TNF-α, p<0.001). This is in contrast to the frequencies detected in mice that received an adenoviral priming, as the frequencies of IL-2 and IFN-γ secreting CD8+ T cells induced were comparable regardless of the adenovirus used (Figure 4A). When comparing the frequency of TNF-α secreting CD8+ T cells, this response was higher in mice immunized with Ad5PP than with Ad5/3PP (p<0.01). Following identification of cytokine producing CD8+ T cells, we determined the quality of the T cell response, defined as the simultaneous production of two or three cytokines with their relative proportion represented by pie charts (Figure 4B). Differences in the proportion of double or triple cytokine producing CD8+ T cells were observed in mice that received either heterologous immunization with recombinant Ad5 in comparison to the PPP immunization regimen (PPAd5 and Ad5PP, p<0.05). Significant differences in the proportion of double or triple cytokine producing CD8+ T cells were also observed in mice that received the boosting immunization with Ad5/3 (PPAd5/3, p<0.05). Although differences in the combined double or triple cytokine producers for all antigens tested were not significant for Ad5/3PP immunized mice, the highest frequency of triple CD8+ T cells producers was observed in this group after ex vivo stimulation with a peptide pool containing the SYVPSAEQI epitope (pool PyLPC, Figure 4B).
Figure 4. Cytokine production and multifuncionality of CD8+ T cells induced by the different prime boost heterologous regimens.
Frequencies of spleen CD8+ T cells producing IL-2, IFN-γ, or TNF-α obtained 5 days after the last immunization with the heterologous regimens, upon stimulation with pools of synthetic peptides: PyLPC (black bars), PyRMC pool 1 (gray bars), and PyRMC pool 2 (white bars). Bars represent mean responses ± standard deviations for five mice per group. (B) To determine the proportions of multifunctional CD8+ T cells, Boolean gate analysis was used to identify and quantify the fraction of the total response of cells that produced three, two, or one cytokine in response to the corresponding antigen. The pie charts summarize the fractions of single, double, or triple producers for indicated groups in the experiment described for panel A. Box contains the regimens that significantly produce two or more cytokines in comparison to the PPP immunization regimen (p<0.05 by one-way ANOVA with Bonferroni’s posttest).
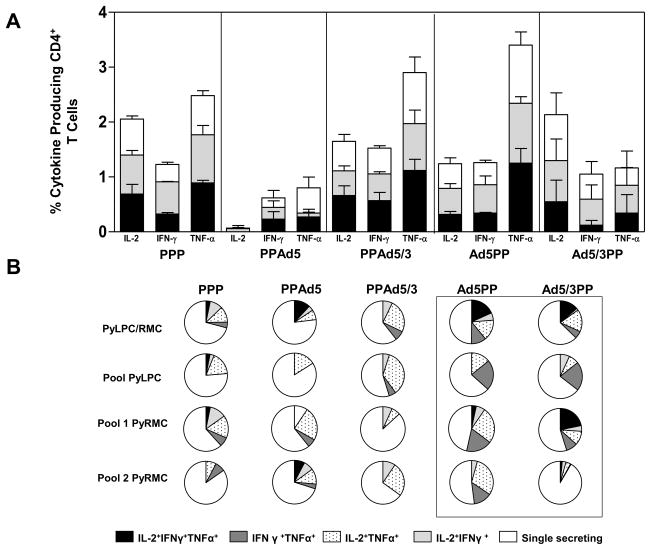
Similar to our observations of CD8+ T cells, high frequencies of cytokine secreting CD4+ T cells were detected in response to the three peptide pools tested in mice immunized with the PPP regimen. Differences in the frequencies of CD4+ T cells specific to the PyLPC/RMC peptide pools were detected between the PPAd5 and PPAd5/3 immunization groups, with higher frequencies of cytokine secreting cells observed in mice that received the heterologous PPAd5/3 regimen with the fiber modified vector (IL-2, p=0.0091; IFN-γ, p=0.0039 and TNF-α, p=0.0078) (Figure 5A). Differences in the proportion of double or triple cytokine producing CD4+ T cells were observed in mice that received heterologous immunizations that included the recombinant Ad5 and Ad5/3 vectors as priming in comparison to the PPP immunization regimen (Ad5PP, p<0.01; Ad5/3PP, p<0.05) (Figure 5B).
Figure 5. Cytokine production and multifuncionality of CD4+ T cells induced by the different prime boost heterologous regimens.
Frequencies of spleen CD4+ T cells producing IL-2, IFN-γ, or TNF-α obtained 5 days after the last immunization with the heterologous regimens, upon stimulation with pools of synthetic peptides: PyLPC (black bars), PyRMC pool 1 (gray bars), and PyRMC pool 2 (white bars). Bars represent mean responses ± standard deviations for five mice per group. (B) To determine the proportions of multifunctional CD4+ T cells, Boolean gate analysis was used to identify and quantify the fraction of the total response of cells that produced three, two, or one cytokine in response to the corresponding antigen. The pie charts summarize the fractions of single, double, or triple producers for indicated groups in the experiment described for panel A. Box contains the regimens that significantly produce two or more cytokines in comparison to the PPP immunization regimen (p<0.05 by one-way ANOVA with Bonferroni’s posttest).
Protective efficacy elicited by immunization with heterologous adenoviral prime-boost immunization regimens
To evaluate the protective immunity induced by the heterologous prime-boost immunization regimens, mice were experimentally challenged with P. yoelii sporozoites thirty days after the final boosting immunization. Parasitemias and hemoglobin levels were determined daily, and protective efficacy was assessed as described above. Mice immunized with empty adenovirus vectors were used as a control. Following challenge, the control group developed high parasitemia and severe anemia that reached the cutoff of 5 g/dL between day 10 and 13 post challenge. All the immunization regimens tested induced protection against sporozoite challenge with significant differences in parasitemia when compared to the group of mice immunized with the empty vectors (p<0.001, Figure 6A and B). The parasitemia and hemoglobin levels of mice in groups that included Ad5 (Figure 6A and C) or the fiber modified Ad5/3 (Figure 6B and D) were comparable.
Figure 6. Protective effect of the prime-boost strategy including recombinant Ad5 or Ad5/3 vectors.
Effect of heterologous prime-boost regimens based on Ad5 (Left Panels) or Ad5/3 (Right Panels) on Parasitemia (A–B) expressed as an area under the curve of Parasitemia vs Time and hemoglobin levels (C–D) expressed as the lowest recorded hemoglobin presented as percentage of the baseline hemoglobin. 20 days after the final immunization mice were challenged with P. yoelii sporozoites. Data are the summary of the results of two independent experiments (n=12 mice). Error bars show standard deviations. Immunization groups were compared to a control group receiving empty adenoviral vectors immunizations using one-way ANOVA and Bonferroni’s Post-test *p<0.05, *p<0.001.
The heterologous immunization regimens that included priming with the adenoviral vectors and boosting with the recombinant protein, Ad5PP and Ad5/3PP regimens, exhibited parasitemias four times lower than that of the PPP or PPAd groups, although these results were not statistically significant. Furthermore, the AdPP regimens induced sterilizing immunity in 66.7% (8/12) of the mice primed with Ad5 and in 41.6% (5/12) of those primed with Ad5/3. There was not a significant difference in the proportion of mice that achieved sterilizing immunity between the Ad5PP and the Ad5/3PP regimens (p=0.21). Of the mice that developed parasitemia, the mean pre-patent period was 6.8 days (± 1.3) for the priming with Ad5, and 7.0 (± 1.3) days for the Ad5/3 priming. In comparison, mice immunized with an empty Ad5 had a mean pre-patent period of 3.6 days (± 1.2), while mice immunized with an empty Ad5/3 had a mean pre-patent period of 3.3 days (± 0.5). The differences of the pre-patent periods between the Ad-Protein regimens and the empty vectors where statistically significant (p<0.001) (Supplementary Figure 1).
Effect of CD4+ or CD8+ T cell depletion on vaccine efficacy
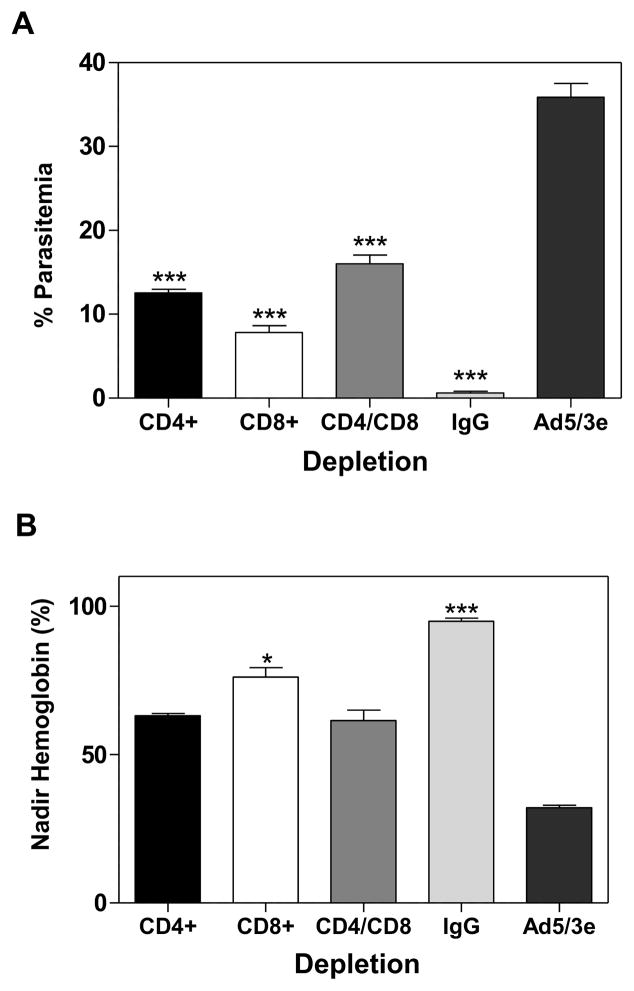
We have previously demonstrated the relevance of antibodies in protection induced by immunization with a homologous prime-boost regimen of the PyLPC/RMC protein (4). In vivo depletion experiments were used to assess the roles of CD4+ and CD8+ T cells in protective efficacy induced by immunization with a heterologous prime-boost regimen. Having shown that immunization with heterologous regimens involving adenovirus priming are more effective, the Ad5/3-PP regimen was selected for characterization of the cellular effector mechanisms involved in protection. CB6F1/J mice were immunized with the Ad5/3PP regimen and four weeks after the last boosting immunization T cells were depleted with either anti-CD4+ (clone GK1.5), anti-CD8+ (clone YTS169.4), or a combination of both monoclonal antibodies. Control mice immunized with Ad5/3-PP received purified polyclonal rat IgG. Antibodies were administered intraperitoneally at 500 μg/dose, and given on days −1 and +2 relative to the day of challenge. The efficiency of cell depletion was monitored by flow cytometry analysis (data not shown). After experimental challenge, all the mice immunized with the empty vector developed high parasitemias and severe anemia between day 10 and 12 after challenge and were euthanized. All mice depleted of CD4+ T cells developed parasitemias that ranged from 12.3 to 15.2% at day 12 (p<0.0001, as compared to Ad5/3PP immunized mice that received normal rat IgG). In the CD8+ depleted group, day 12 parasitemia ranged between 5.4 and 11.5%, significantly higher than the parasitemia of Ad5/3-PP immunized mice receiving normal IgG (p<0.001) (Figure 7A). Similarly, in the CD4+/CD8+ depleted group, all mice had peaks of parasitemias that ranged from 9.5 to 18.3% at day 12 (p<0.001). All the PyLPC/RMC immunized mice treated with normal rat IgG survived the challenge and presented a day 12 parasitemia ranging between 0.2 and 1.5%. Consistent with parasitemias, mice in the CD4+, CD8+, and CD4+/CD8+ depleted groups also had reduction in hemoglobin levels in comparison to baseline levels (p<0.001) (Figure 7B).
Figure 7. T cell depletion effect on protective efficacy in mice immunized with the Ad5/3PP regimen.
Effects of CD4+ or CD8+ T cell depletion in CB6F1/J mice immunized with a heterologous regimen consisting in priming with Ad5/3 and boosting with PyLPC/RMC formulated in Montanide ISA 51. Mice were treated the day before challenge and again on day 2 after challenge using 500 μg of GK1.5 anti-CD4 monoclonal antibody, YTS169.4 anti-CD8 monoclonal antibody, or a combination of both. Control mice immunized with the same regimen were treated with normal rat IgG. As a control for infection, empty adenoviral vectors immunized mice were included (Ad5/3e). Bars show the mean of parasitemias at the peak expressed as percentage of parasitemia, error bars indicate SD (A) or the mean hemoglobin reduction at the nadir expressed as a percentage of the baseline hemoglobin, error bars indicate SD (B). Data are representative of two independent experiments (n=6 mice). *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA with Bonferroni’s posttest in comparison to mice immunized with empty adenovectors.
Neutralization capabilities of anti-Ad5 antibodies in the immunogenicity of heterologous Ad-prime protein-boost regimens
To assess the ability of anti-Ad5 antibodies to neutralize the chimeric Ad5/3 vector, we generated anti-vector antibodies in mice by immunization with an empty Ad5 vector. Anti-Ad5 antibodies neutralized the Ad5 vector but exhibited a lower activity in neutralizing the fiber modified Ad5/3 vector, requiring twice the amount of antibodies that were required to neutralize Ad5 (p=0.006) (Figure 8A). Consistent with this observation, when mice previously sensitized with Ad5 were immunized with the Ad5/3PP or the Ad5PP regimens, the proportion of CD8+ T cells able to recognize the SYVPSAEQI tetramer was significantly higher in the Ad5/3PP regimen but not in the Ad5PP regimen when these regimens were compared to the control group receiving a priming with an empty Ad5 and boosted with Montanide ISA 51 (Figure 8B). No differences between the regimens were found in antibody titers against PyLPC/RMC (Figure 8C). When the CD4+ and CD8+ cytokine secreting T cells were assessed after ex vivo stimulation with PyLPC/RMC, we observed a significantly lower frequency of IFN-γ and IL-2 secreting CD4+ T cells and a significantly lower frequency of IFN-γ, IL-2 and TNF-α secreting CD8+ T cells in mice immunized with Ad5PP when compared to Ad5/3PP (Figure 8D and E).
Figure 8. Effect of Anti-Ad5 antibodies in the immunogenicity of Ad5 and Ad5/3.
(A) Neutralization of Ad5 (Closed Bars) or Ad5/3 (Open Bars) by sera of mice immunized with Ad5. Viruses at an MOI of 10 were incubated with 5μl of sera from Ad5 immunized CB6F1/J mice before infecting HeLa cells plated in 96-well plates. The infectivity was determined by measurement of the luciferase transgene. The results present the reciprocal mean neutralization titers able to neutralize 50% of the luciferase activity ** p<0.01 by two tailed unpaired Student’s T test. The experiments were done in triplicate. (B) Specific SYVPSAEQI/APC Tet+ CD8+ T cells induced by immunization with Ad5/33PP or Ad5PP regimens in mice (n=5 per group) previously immunized with empty Ad5 to induce neutralizing antibodies. Tetramer recognition was assessed 10 days after the final protein boost and is expressed as arithmetic mean values ± standard deviations (SD). **p<0.01 by Kruskal-Walllis with Dunns’ post-test comparsions are presented between the immunization groups and the control group. (C) Anti-PyLPC/RMC antibody titers measured 20 days after the third and final immunization. Endpoint ELISA titers at 0.5 optical density were determined using the recombinant protein PyLPC/RMC and serum samples from mice immunized with the different heterologous regimens and expressed as arithmetic mean values ± standard deviations (SD). (D) Frequencies of CD4+ and (E) CD8+ IFN-γ, IL-2 or TNF-α secreting T cells after stimulation with PyLPC/RMC. Spleens were obtained from previously Ad5 sensitized mice, after immunization with the heterologous regimens. Bars represent mean responses ± standard deviations for five mice per group.
Effect of the adenoviral dose in the immunogenicity and protective efficacy of Ad prime – protein boost heterologous regimens
Higher doses of recombinant Ad vectors, compared to those reported here, have been reported for vaccine candidates in other experimental models (16, 17). We therefore decided to immunize CB6F1/J mice with the heterologous Ad5PP and Ad5/3PP regimens with the standard 107 v.p. dose (denominated Ad5PP-S, Ad5/3PP-S) and a high 1010 v.p dose (denominated Ad5PP-H, Ad5/3PP-H). The recognition of the SYVPSAEQI tetramer by CD8+ T cells 20 days after the last immunization was significantly lower in the groups that received high adenoviral doses, irrespective of the vector used (Figure 9A). The dual expression of CD49d and CD11a has been used to define antigen experienced T cells as a surrogate marker of CD4+ T cell activation (13). Similar to the tetramer recognition in CD8+ T cells, when we assessed CD49d and CD11a expression we found that the proportion of antigen experienced CD4+ T cells was significantly lower when the high adenoviral doses were used for priming when compared to the standard 107 v.p. dose.
Figure 9. Effect of the adenoviral dose in the immunogenicity and protective efficacy of heterologous Ad Prime – Protein boost regimens.
(A) Specific SYVPSAEQI/APC Tet+ CD8+ T cells induced by immunization with Ad5/3PP or Ad5PP using the standard adenoviral dose 107 v.p. (S) or a high adenoviral dose of 1010 v.p. (H) in mice (n=5 per group). Tetramer recognition was assessed 20 days after the final protein boost and is expressed as arithmetic mean values ± standard deviations (SD). **p<0.01 by Mann-Whitney test comparisons are presented between the different doses of the same adenovirus. (B) Antigen experienced CD4+ T cells (CD49d+ and CD11a+) induced by immunization with Ad5/3PP or Ad5PP using the standard or a high adenoviral dose in mice (n=5 per group) *p<0.05 **p<0.01 by Mann-Whitney test. (C–D) Effect of the adenoviral priming doses in protective efficacy after experimental challenge with P. yoelii sporozoites. (C) Parasitemia expressed as the area under the curve of Parasitemia vs Time and (D) hemoglobin levels expressed as the lowest recorded hemoglobin presented as percentage of the baseline recording. (n=5 mice). Error bars show standard deviations. Immunization groups were compared to a control group receiving no immunizations using one-way ANOVA and Bonferroni’s Post-test *p<0.05, **p<0.01.
When the cytokine expression was evaluated in splenocytes obtained from mice immunized with the different regimens 5 days after the final immunization, we observed a significantly a higher frequency of IFN-γ secreting CD8+ T cells in both the Ad5 and Ad5/3 immunized mice with standard priming dose, and a higher frequency of IFN-γ secreting CD4+ T cells in mice immunized with the Ad5/3 regimen with standard priming dose (Supplementary Figure 2). Although the only other statistically significant difference was a higher frequency of TNF-α secreting CD4+ T cells induced by the Ad5PP regimen with standard priming, it is important to note that the frequency of cytokine secreting cells tended to be higher in the standard dose regimens when compared to the high dose regimens (Supplementary Figure 2).
Protection was assessed as the AUC of parasitemia vs time, and consistent with our previous observations the standard dose regimens showed a significant reduction of the parasitemia when compared to a control group receiving empty adenoviral vectors (Figure 9C). Interestingly, we observed a loss of protection with the high adenoviral doses and in the Ad5/3PP group parasitemias were higher than those recorded in naïve mice. When the hemoglobin was assessed at the nadir, only the Ad5/3PP-S group was able to significantly control the hemoglobin drop associated to the infection (Figure 9D).
Expression of PD-1 and memory markers on T cells after immunization with high doses of Ad vectors
The expression of PD-1 has been associated with functional exhaustion of T cells (18). We examined the effect of high doses of adenovirus vectors on PD-1 expression to assess if T cell dysfunction can be associated with the poor efficacy observed after challenge. PD-1 expression was similar in all regimens tested, with no statistical differences between the regimens (Data not shown).
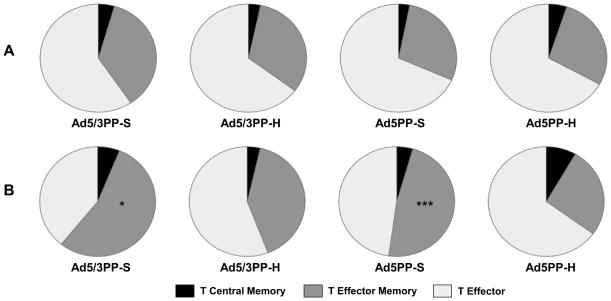
The memory phenotype was assessed in PBMC by measuring the expression of CD127 and CD62L. The expression of CD62L (L-selectin) and CD127 (IL-7R α-chain) allow us to define 3 different memory populations: 1) Central memory T cells (CD62L+CD127+), 2) Effector memory T cells (CD62L−CD127+), and 3) Effector T cells (CD62L−CD127−) (19, 20). When we analyzed the different memory subsets in antigen experienced CD4+ T cells (Figure 10A) and in SYVPSAEQI tetramer+ CD8+ T cells (Figure 10B), we observed a significantly larger population of CD8+ effector memory T cells in the standard dose group when compared to the high dose groups in response to both viral vectors.
Figure 10. Malaria specific memory T cell induction by different adenoviral priming regimens.
20 days after the final immunization memory T cells subpopulations were defined using the expression of CD62L (L-selectin) and CD127 (IL-7R α-chain). The population of T cells was then classified into central memory T cells (CD62L+ CD127+), effector memory T cells (CD62L− CD127+) and effector T cells (CD62L− CD127−). Results are presented as the proportion of the cells corresponding to the total population of (A) antigen experienced CD4+ T cells or (B) Specific SYVPSAEQI/APC Tet+ CD8+ T cells. Comparison between the individual vectors standard and high dose groups (n=5 mice per group) were performed by Mann-Whitney test *p<0.05, **p<0.001.
Discussion
A second generation of malaria vaccines should be able to induce long lasting immunity and robust T cell responses. To address this, we have developed a chimeric protein PyLPC/RMC that elicits protection through strong antibody and CD4+ T cell responses against pre-erythrocytic and erythrocytic P. yoelii stages (4). To induce an effective CD8+ T cell mediated immunity with our construct, the use of adenovirus was considered as the best option given their high immunogenicity and safety profiles (21–24). In malaria, Ad5 expressing P. yoelii CSP was able to protect mice by inducing a CD8+ IFN-γ mediated T cell response (25). This vector also induces a better cellular immunity against the conserved CSP regions than RTS,S/AS01B, the only malaria vaccine tested in phase III clinical trials (26). Nonetheless, the high prevalence of neutralizing antibodies against this vector, especially in malaria endemic areas, created the need to explore vector alternatives (27).
Rare human adenovirus serotypes or simian adenoviruses can be considered an alternative given the low prevalence of neutralizing antibodies to these viruses. However, these viruses appear to be less immunogenic than Ad5 (6, 26, 28, 29). Since the pre-existing immunity towards Ad5 is mainly directed to the fiber (30), we decided to use a chimeric Ad5/3 vector, in which the Ad5 fiber knob region was replaced with the orthologous region from the rare human adenovirus, Ad3 (31). In this study, mice previously immunized with empty Ad5 vectors produced antibodies that were able to neutralize Ad5 much more efficiently than Ad5/3. The impact of pre-existing anti-Ad5 antibodies was also tested in vivo with the Ad5/3PP regimen showing a more efficient induction of cellular immune response in comparison to Ad5 sensitized mice immunized with the Ad5PP regimen. Our results are in contrast with data reported by Bruder and colleagues working with chimeric Ad5 vectors containing all nine hypervariable regions of the hexon protein derived from Ad43. Those vectors were able to induce T cell responses in mice against CSP, but were not neutralized by neutralizing antibodies induced by previous Ad5 immunizations, Nonetheless, anti-fiber antibodies were able to neutralize them in vitro (32). Both observations could indicate that although anti-fiber or anti-hexon antibodies alone are able to neutralize Ad vectors in vitro, a synergy between anti-hexon and anti-fiber antibodies is necessary to neutralize Ad vectors in vivo.
Here we also demonstrate that immunogenicity of recombinant Ad5/3 is no different than that of recombinant Ad5, as no significant differences were found between Ad5/3 and Ad5 in antibody responses and cellular production of IFN-γ, IL-2, and TNF-α after stimulation with peptides pools representing different components of the PyLPC/RMC transgene. Also it is likely that the Ad5/3 immunogenicity could be underrated by the model’s limitations, since Ad5/3 is able to infect DCs in humans and non-human primates through CD46 (33, 34), a receptor not expressed in female mice. Ad5/3 also exhibits tropism for human dendritic cells (DC) through CD80 and CD86 (35), and significantly higher tropism for macaque DC in comparison to Ad5 (Blackwell, J. and Curiel, D., unpublished data).
During the course of this study, we have also confirmed the reports that regimens comprising homologous Ad immunizations exhibit reduced efficacy when compared to heterologous regimens due to the induction of anti-vector immunity (36, 37), as we demonstrated that homologous immunization regimens with recombinant Ad5 or Ad5/3 vectors display reduced protective efficacy when compared with a protein only homologous regimen. To avoid the potential deleterious effect of the induction of anti-vector immunity by immunization, we tested several immunization regimens that involved a single Ad vector immunization. The optimized immunization regimen we describe here are in agreement with previous reports that highlighted the relevance of Ad vectors as a robust priming agent in heterologous immunization regimens that involve recombinant proteins (22, 38).
When we assessed the antibody response, both the Ad5PP and the Ad5/3PP immunization regimens induced significantly lower anti-PyLPC/RMC titers than the PPP, PPAd5 and PPAd5/3 regimens. No differences in antibodies affinity were observed, suggesting that the use of Ad vectors did not affect the antibody functionality. A heterologous regimen that combined a recombinant Ad35 expressing CSP for priming and RTS,S/AS01B for boosting also produced lower antibody titers in comparison to homologous prime-boost immunization regimen with RTS,S/AS01B (22). In contrast, Douglas et al. reported a vaccine regimen consisting of priming immunization using the simian AdCh63 and a protein boost, that was able to induce higher antibodies titers when compared with a homologous regimen consisting of two immunizations with a P. falciparum MSP-1 protein-based vaccine (39). These differences can be explained by the better antibody profile induced by erythrocytic stage antigens like MSP-1 (40).
In addition to the antibody response, cellular immunity also plays a critical role in malaria protection. IFN-γ secreting CD8+ T cells are one of the effector mechanisms to control hepatocyte infection, inducing the production of nitric oxide in liver cells for the destruction of the parasite or the infected hepatocytes (41, 42). Significantly higher frequencies of PyLPC/RMC-specific CD8+ T cells were found in mice immunized with the regimens that involved boosting with the recombinant Ad vectors when compared with the PPP regimen. Although the CD8+ T cell responses induced by the regimens that involved priming with the recombinant Ad vectors were higher than the PPP regimen, such responses were lower in comparison to the regimens involving recombinant Ad vector for boosting immunization. It could be hypothesized that our protein induces CD8+ responses susceptible to be boosted by the adenovirus vector, but when used for boosting immunizations the protein is only able to sustain the responses obtained by Ad vectors. Similarly, Rodriguez et al, showed that using recombinant Ad35 as a priming strategy to deliver the Liver Stage Antigen 1 (LSA-1) significantly enhanced the number of IFN-γ producing CD4+ T cells, while enhancing IFN-γ producing CD8+ T cells when used for boosting (38). This effect is also seen in a tuberculosis model using BCG for priming immunization and a recombinant Ad5 expressing the M. tuberculosis Antigen 85A as boosting in cattle (43).
The use of Ad vectors in vaccination regimens have shown both improved cellular response and induction of multifunctional T cells when compared with protein immunizations (44). Protection in malaria has also been correlated with multifunctional CD4+ T cells in volunteers immunized with sporozoites while taking chloroquine prophylaxis (45). Meanwhile the association between CD8+ multifunctional T cells and protection in malaria is not clear (46, 47). Ad5/3 priming was able to induce the highest number of triple cytokine producing cells in both CD4+ and CD8+ subsets and together with the Ad5PP regimen it was able to induce significantly more double and triple cytokine producing CD4+ and CD8+ T cells when compared with the protein only regimen. These results are congruent with a phase I Mycobacterium tuberculosis vaccine clinical trial where an Ad5 vector presenting Antigen 85A was able to induce multifunctional CD4+ and CD8+ T cells in humans previously vaccinated with BCG (48).
Priming with recombinant Ad5 or Ad5/3 and boosting with PyLPC/RMC were the most efficacious heterologous prime-boost vaccination regimens, as revealed by the higher proportion of mice with sterilizing immunity, lower parasite loads and lower reduction of hemoglobin when compared with the other immunization regimens. Despite the higher numbers of CD8+ T cells induced by the PP-Ad regimens, the protective effect was lower than both the Ad-PP and the PPP regimens. This can be explained by the better quality of T cells and antibodies (as observed by CD4+ T cell multifunctionality and antibody affinity) obtained with the Ad priming when compared to protein priming (49). Ad vectors also maintain a transgene expression for periods longer than a year which are able to maintain sustained frequencies of transgene product-specific CD8+ T cells (50), this effect can also explain the higher protective efficacy obtained with a heterologous Ad prime – protein boost regimen when compared with an homologous protein regimen.
Depletion of T cells in the Ad5/3PP immunization regimen showed that CD8+ T cells have an impact on both parasite load and hemoglobin levels when compared to mice immunized with the same regimen that received normal rat IgG as a control, an effect that was not observed with previous experiment with a homologous protein regimen (4). Consistent with our previous results, CD4+ T cell depletion also led to a significant increase in parasite load and a decrease in hemoglobin levels. The predominant role of CD4+ in our studies could be explained by the findings of Provine et al, which demonstrated that CD4+ T cells are required by CD8+ T cells for successful priming, effector differentiation, contraction regulation, and protection after immunization with Ad vectors (51). CD4+ T cells also have several roles in anti-Plasmodium immunity, being able to control parasite development by IFN-γ mediated nitric oxide production and TNF-α acting on the infected hepatocytes (52, 53). An IFN-γ independent active elimination of malaria parasites from hepatocytes has also been reported (54, 55). This elimination occurs both in vivo an in vitro, and is mediated by a cytotoxic effect via granule-mediated exocytosis of perforin and granzymes (56). Passive transfers of these cells are able to induce protection (57) and humans immunized with irradiated sporozoites or the (T1BT*)4-P3C malaria peptide containing immunodominant T cell epitopes of P. falciparum CSP are able to induce similar CD4+ T cell subsets (56). The T cell depletion assays also showed that mice depleted of both CD4+ and CD8+ T cells exhibited lower levels of parasitemia in comparison with mice immunized with an empty adenovirus, confirming the protective role of the anti-PyLPC/RMC antibodies (4). Draper et al. similarly reported a vaccine candidate based on MSP-1 delivered using priming with chimpanzee Ad63 followed by a boosting with a recombinant modified vaccinia Ankara (MVA) with the ability of maintain its protective effect after T cell depletion (58).
Doses of recombinant adenovirus vectors higher than 107 v.p. have been able to enhance the cellular response against the transgene product when delivered by rare human adenoviral vectors (59, 60). Nonetheless, in our model priming with a 1010 vp of Ad5 or Ad5/3 recombinant vectors induced lower tetramer specific CD8+ T cells and antigen experienced CD4+ T cells. This observation was consistent with the loss of protective efficacy in mice primed with the high Ad dose when compared to that including priming with the standard 107 v.p. dose. T cell exhaustion could be responsible for this effect. However, we were unable to detect differences in the expression of PD-1 on CD4+ or CD8+ T cells in mice immunized with high or standard doses of recombinant Ad vectors. In a recent study on CD4+ T cell exhaustion, Han et al. proposed that high antigen doses are related with a decline in T cell functionality and a reduced memory capacity in a process similar to T cell exhaustion. The effect in the reported model was not associated with PD-1 but with an impaired Jun phosphorylation in response to TCR engagement (61). It may be likely that a similar mechanism is responsible for our findings.
It has been suggested that the immunizations with Ad vectors can induce different T cell responses ranging from the expansion and the induction of effector T cells to the loss of functional responses (62). This broad range of immune responses is ultimately associated to the dosage, with effective adenoviral doses showing differences at only 1 to 2 log (62). In our study, we tested a dose 3 log higher than the efficacious dose and found that instead of improving efficacy the protection was abrogated, further confirming that Ad vectors do not exhibit a linear dose – effect correlation (62, 63). Krebs and colleagues, also found a similar effect with the use of an Ad vector expressing β-galactosidase as a transgene (62), their results showed that Ad vectors could induce a tolerogenic effect at high doses. This effect has also been confirmed by other groups (64) and in our study with the Ad5/3PP-H group showing higher parasitemias than the control group. In addition, Krebs et al, proposed that Ad vectors can deliver their transgene to professional APCs in the secondary lymphoid organs leading to DC maturation and at the optimal dose are able to induce effector CTL. Nonetheless, high doses would still stimulate the APCs in secondary lymphoid organs long after the inflammatory response mediated by the vector waned leading to transgene presentation by APCs that lack the appropriate costimulatory molecules preventing optimal T cell activation (62). Based on the lack of a complete understanding of adenoviral induced exhaustion, further studies are required to define the molecular mechanisms behind the effect of the adenoviral doses on the induction of tolerogenic APCs.
The effect of cellular dysfunction induced by high doses is not limited to Ad vectors. Wherry et al, using an MVA platform showed that there is a threshold for the optimal level of epitope necessary to induce memory responses, with doses higher than optimal inducing a qualitatively inferior memory cell population (65). In this study they demonstrated that the levels of the epitope of interest in the priming determines the memory population. This is consistent with our study in which we observed that the magnitude of the priming dose determines the quality of the cellular responses in terms of antigen specific CD4+ and CD8+ T cells.
Differences in the induction of tetramer specific CD8+ memory T cells are showed here withgnificantly higher frequency of effector memory T cells in both standard dose groups. These results could be explained by the trend that stronger and longer antigenic stimuli tend to increase the number of cells that differentiate into effector cells, while weaker signals are able to induce the differentiation towards central and effector memory cells (66). In natural infections, high antigen levels are related with the production of single cytokine producing cells, loss of multifunctionality, and the progression to chronic infections (67). Additionally, this loss of multifunctionality is also related to impaired development of memory T cells (68).
Our finding that effector memory CD8+ T cells able to recognize a hepatic antigen (the SYVPSAEQI tetramer is specific for CSP) is related with protection is consistent with the report of Reyes-Sandoval et al, that demonstrated that high frequency of CD8+ T cells was a correlate of protection for liver malaria using a different Plasmodium antigen, the thrombospondin related adhesion protein (TRAP), and a different malaria parasite (P. berghei) (19). The need for effector memory cells in protection against pre-erythrocytic malaria can be explained by the ability of these cells to enter tissues to create a resident T cell memory population which could react much more efficiently than a central memory T cell population resident in a secondary lymphoid organ (19). The fact that this population is related with protection with two different hepatic antigens and two different Plasmodium species makes for a strong argument that CD8+ effector memory T cells are a correlate of protection for liver malaria.
In summary, we have developed a chimeric adenovirus vector, able to circumvent anti-Ad5 immunity. This fiber modified Ad5/3 recombinant vector enhances cellular and antibody responses when used as a part of a prime-boost immunization regimen with a chimeric multistage protein targeting both the hepatic and the erythrocytic stages of P. yoelii. Such responses are associated with enhanced efficacy. This novel prime-boost regimen was able to induce a better cellular anamnestic profile when low doses of adenovirus were used for priming, demonstrating that the protective efficacy of Ad vectors does not follow a linear correlation but there is a threshold of efficacy where higher doses induce T cell dysfunction and loss of anamnestic potential. Our results support further studies with this vector for malaria vaccine development.
Supplementary Material
Acknowledgments
We thank Jessica N. McCaffery for critical reading of the manuscript.
Footnotes
This research was supported by National Institutes of Health, NIAID grant R56-AI103382-01A1, R21-AI094402-01 and R21-AI095718-01, and the Yerkes National Primate Research Center Base Grant No RR00165 awarded by the National Center for Research Resources of the National Institutes of Health.
References
- 1.World Health Organization. World malaria report 2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 2.Singh B, Cabrera-Mora M, Jiang J, Galinski M, Moreno A. Genetic linkage of autologous T cell epitopes in a chimeric recombinant construct improves anti-parasite and anti-disease protective effect of a malaria vaccine candidate. Vaccine. 2010;28:2580–2592. doi: 10.1016/j.vaccine.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Flannery LM, Cabrera-Mora M, Jiang JL, Moreno A. Recombinant peptide replicates immunogenicity of synthetic linear peptide chimera for use as pre-erythrocytic stage malaria vaccine. Microbes and Infection. 2009;11:83–91. doi: 10.1016/j.micinf.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh B, Cabrera-Mora M, Jiang J, Moreno A. A hybrid multistage protein vaccine induces protective immunity against murine malaria. Infection and immunity. 2012;80:1491–1501. doi: 10.1128/IAI.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuldt NJ, Amalfitano A. Malaria vaccines: focus on adenovirus based vectors. Vaccine. 2012;30:5191–5198. doi: 10.1016/j.vaccine.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. Journal of virology. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley RR, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. Journal of virology. 2012;86:625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereboev AV, Nagle JM, Shakhmatov MA, Triozzi PL, Matthews QL, Kawakami Y, Curiel DT, Blackwell JL. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol Ther. 2004;9:712–720. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Dmitriev I, Kashentseva E, Seki T, Wang M, Curiel DT. Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. Journal of virology. 2002;76:12775–12782. doi: 10.1128/JVI.76.24.12775-12782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 2005;7:1324–1337. doi: 10.1016/j.micinf.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Caro-Aguilar I, Rodriguez A, Calvo-Calle JM, Guzman F, De la Vega P, Patarroyo ME, Galinski MR, Moreno A. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect Immun. 2002;70:3479–3492. doi: 10.1128/IAI.70.7.3479-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert opinion on biological therapy. 2009;9:1521–1531. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- 15.Krasnykh V, Mikheeva G, Douglas J, Curiel D. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. Journal of virology. 2013;87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, Iampietro MJ, Barouch DH. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. Journal of virology. 2013;87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, Hill AV. CD8+ T effector memory cells protect against liver-stage malaria. Journal of immunology. 2011;187:1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. Journal of immunology. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine. 1998;16:1812–1817. doi: 10.1016/s0264-410x(98)00181-9. [DOI] [PubMed] [Google Scholar]
- 22.Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, Cobb M, Burge JR, Larson D, Ware LA, Demoitie MA, Weverling GJ, Bayat B, Custers JH, Dubois MC, Cohen J, Goudsmit J, Heppner DG., Jr Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infection and immunity. 2007;75:2283–2290. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez A, Mintardjo R, Tax D, Gillissen G, Custers J, Pau MG, Klap J, Santra S, Balachandran H, Letvin NL, Goudsmit J, Radosevic K. Evaluation of a prime-boost vaccine schedule with distinct adenovirus vectors against malaria in rhesus monkeys. Vaccine. 2009;27:6226–6233. doi: 10.1016/j.vaccine.2009.07.106. [DOI] [PubMed] [Google Scholar]
- 24.O’Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, Rowland R, Sheehy SH, Poulton ID, Hutchings C, Todryk S, Andrews L, Folgori A, Berrie E, Moyle S, Nicosia A, Colloca S, Cortese R, Siani L, Lawrie AM, Gilbert SC, Hill AV. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205:772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruna-Romero O, Rocha CD, Tsuji M, Gazzinelli RT. Enhanced protective immunity against malaria by vaccination with a recombinant adenovirus encoding the circumsporozoite protein of Plasmodium lacking the GPI-anchoring motif. Vaccine. 2004;22:3575–3584. doi: 10.1016/j.vaccine.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Shott JP, McGrath SM, Pau MG, Custers JH, Ophorst O, Demoitie MA, Dubois MC, Komisar J, Cobb M, Kester KE, Dubois P, Cohen J, Goudsmit J, Heppner DG, Stewart VA. Adenovirus 5 and 35 vectors expressing Plasmodium falciparum circumsporozoite surface protein elicit potent antigen-specific cellular IFN-gamma and antibody responses in mice. Vaccine. 2008;26:2818–2823. doi: 10.1016/j.vaccine.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 27.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. Journal of virology. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, Giles-Davis W, Wilson JM, Ertl HC. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. Journal of immunology. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 30.Cheng C, Gall JG, Nason M, King CR, Koup RA, Roederer M, McElrath MJ, Morgan CA, Churchyard G, Baden LR, Duerr AC, Keefer MC, Graham BS, Nabel GJ. Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. Journal of virology. 2010;84:630–638. doi: 10.1128/JVI.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawakami Y, Li H, Lam JT, Krasnykh V, Curiel DT, Blackwell JL. Substitution of the Adenovirus Serotype 5 Knob with a Serotype 3 Knob Enhances Multiple Steps in Virus Replication. Cancer Res. 2003;63:1262–1269. [PubMed] [Google Scholar]
- 32.Bruder JT, Semenova E, Chen P, Limbach K, Patterson NB, Stefaniak ME, Konovalova S, Thomas C, Hamilton M, King CR, Richie TL, Doolan DL. Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses. PloS one. 2012;7:e33920. doi: 10.1371/journal.pone.0033920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyler MA, I, Ulasov V, Borovjagin A, Sonabend AM, Khramtsov A, Han Y, Dent P, Fisher PB, Curiel DT, Lesniak MS. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5:2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 34.Ulasov IV, Tyler MA, Zheng S, Han Y, Lesniak MS. CD46 represents a target for adenoviral gene therapy of malignant glioma. Human gene therapy. 2006;17:556–564. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- 35.van de Ven R, Lindenberg JJ, Oosterhoff D, van den Tol MP, Rosalia RA, Murakami M, Everts M, Scheffer GL, Scheper RJ, de Gruijl TD, Curiel DT. Selective Transduction of Mature DC in Human Skin and Lymph Nodes by CD80/CD86-targeted Fiber-modified Adenovirus-5/3. [Miscellaneous Article] Journal of Immunotherapy. 2009 Nov-Dec;32:895–906. doi: 10.1097/CJI.0b013e3181b56deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert SC, Schneider J, Hannan CM, Hu JT, Plebanski M, Sinden R, Hill AV. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–1045. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 37.Ophorst OJ, Radosevic K, Havenga MJ, Pau MG, Holterman L, Berkhout B, Goudsmit J, Tsuji M. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infection and immunity. 2006;74:313–320. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez A, Goudsmit J, Companjen A, Mintardjo R, Gillissen G, Tax D, Sijtsma J, Weverling GJ, Holterman L, Lanar DE, Havenga MJ, Radosevic K. Impact of recombinant adenovirus serotype 35 priming versus boosting of a Plasmodium falciparum protein: characterization of T- and B-cell responses to liver-stage antigen 1. Infect Immun. 2008;76:1709–1718. doi: 10.1128/IAI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–7178. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh IB, Choi HK, Lee SW, Woo SK, Kang HY, Won YD, Cho M, Lim CS. Reactivity of sera from cases of Plasmodium vivax malaria towards three recombinant antigens based on the surface proteins of the parasite. Ann Trop Med Parasitol. 2003;97:481–487. doi: 10.1179/000349803235002498. [DOI] [PubMed] [Google Scholar]
- 41.Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. The Journal of experimental medicine. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, Raney JJ, Aniagolu JU, Green SJ. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. The Journal of experimental medicine. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing Mycobacterial antigen 85A and Mycobacterium bovis BCG. Infection and immunity. 2006;74:1416–1418. doi: 10.1128/IAI.74.2.1416-1418.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, Dicks MD, Faber BW, de Cassan SC, Folgori A, Nicosia A, Gilbert SC, Hill AV. Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 45.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 46.Sedegah M, Hollingdale MR, Farooq F, Ganeshan H, Belmonte M, Kim Y, Peters B, Sette A, Huang J, McGrath S, Abot E, Limbach K, Shi M, Soisson L, Diggs C, Chuang I, Tamminga C, Epstein JE, Villasante E, Richie TL. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes. PloS one. 2014;9:e106241. doi: 10.1371/journal.pone.0106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infection and immunity. 2010;78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A, Yin C, Heriazon A, Damjanovic D, Puri L, Hamid J, Xie F, Foley R, Bramson J, Gauldie J, Xing Z. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Science translational medicine. 2013;5:205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 49.de Cassan SC, Forbes EK, Douglas AD, Milicic A, Singh B, Gupta P, Chauhan VS, Chitnis CE, Gilbert SC, Hill AV, Draper SJ. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. Journal of immunology. 2011;187:2602–2616. doi: 10.4049/jimmunol.1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, Lopez-Camacho C, Wherry EJ, Ertl HC. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provine NM, Larocca RA, Penaloza-MacMaster P, Borducchi EN, McNally A, Parenteau LR, Kaufman DR, Barouch DH. Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses. Journal of immunology. 2014;192:5214–5225. doi: 10.4049/jimmunol.1302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Good MF, Doolan DL. Immune effector mechanisms in malaria. Current opinion in immunology. 1999;11:412–419. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 53.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke ED, Hoffman SL. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. Journal of immunology. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 54.Mazier D, Renia L, Nussler A, Pied S, Marussig M, Goma J, Grillot D, Miltgen F, Drapier JC, Corradin G, et al. Hepatic phase of malaria is the target of cellular mechanisms induced by the previous and the subsequent stages. A crucial role for liver nonparenchymal cells. Immunology letters. 1990;25:65–70. doi: 10.1016/0165-2478(90)90093-6. [DOI] [PubMed] [Google Scholar]
- 55.Renia L, Grillot D, Marussig M, Corradin G, Miltgen F, Lambert PH, Mazier D, Del Giudice G. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. Journal of immunology. 1993;150:1471–1478. [PubMed] [Google Scholar]
- 56.Frevert U, Moreno A, Calvo-Calle JM, Klotz C, Nardin E. Imaging effector functions of human cytotoxic CD4+ T cells specific for Plasmodium falciparum circumsporozoite protein. International journal for parasitology. 2009;39:119–132. doi: 10.1016/j.ijpara.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. The Journal of experimental medicine. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, Hill F, Hill AV. Effective induction of high-titer antibodies by viral vector vaccines. Nature medicine. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee EG, Blattman JN, Kasturi SP, Kelley RP, Kaufman DR, Lynch DM, La Porte A, Simmons NL, Clark SL, Pulendran B, Greenberg PD, Barouch DH. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forbes EK, de Cassan SC, Llewellyn D, Biswas S, Goodman AL, Cottingham MG, Long CA, Pleass RJ, Hill AV, Hill F, Draper SJ. T cell responses induced by adenoviral vectored vaccines can be adjuvanted by fusion of antigen to the oligomerization domain of C4b-binding protein. PLoS One. 2012;7:e44943. doi: 10.1371/journal.pone.0044943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20453–20458. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. Journal of immunology. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 63.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. Journal of immunology. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- 64.Fields PA, Armstrong E, Hagstrom JN, Arruda VR, Murphy ML, Farrell JP, High KA, Herzog RW. Intravenous administration of an E1/E3-deleted adenoviral vector induces tolerance to factor IX in C57BL/6 mice. Gene Ther. 2001;8:354–361. doi: 10.1038/sj.gt.3301409. [DOI] [PubMed] [Google Scholar]
- 65.Wherry EJ, McElhaugh MJ, Eisenlohr LC. Generation of CD8(+) T cell memory in response to low, high, and excessive levels of epitope. Journal of immunology. 2002;168:4455–4461. doi: 10.4049/jimmunol.168.9.4455. [DOI] [PubMed] [Google Scholar]
- 66.MMO, Stephens R. Early Decision: Effector and Effector Memory T Cell Differentiation in Chronic Infection. Current immunology reviews. 2013;9:190–206. doi: 10.2174/1573395509666131126231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. Journal of immunology. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 68.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews Immunology. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.