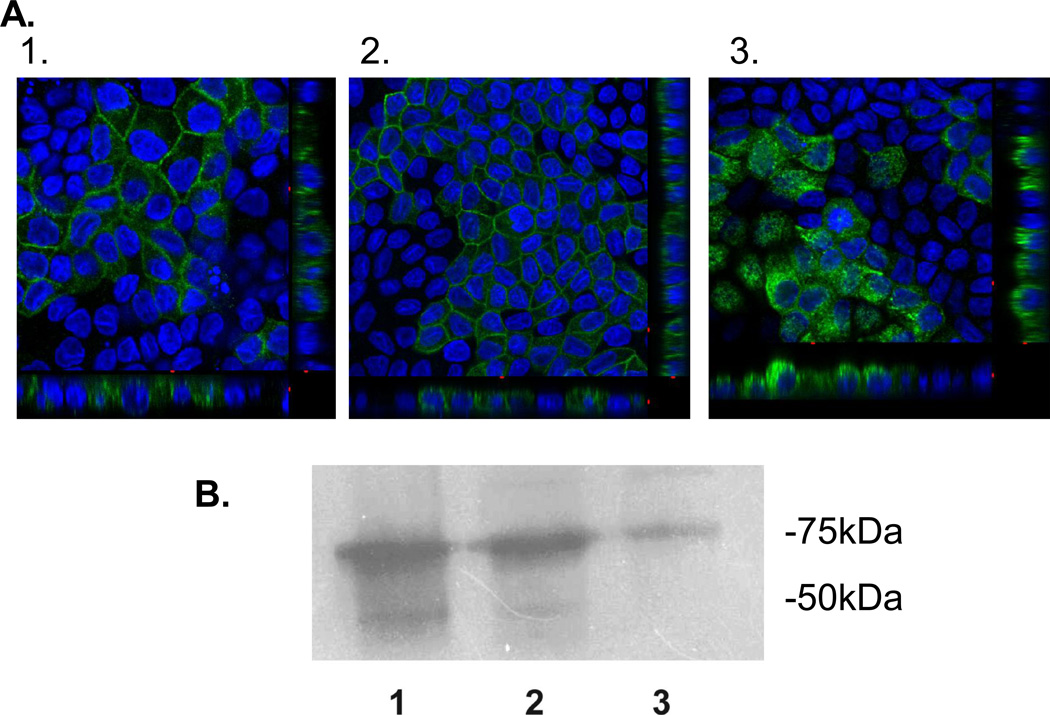
Figure 4. hGLUT9A chimeric proteins show an inability of the GLUT9A N-terminus to redirect an apical sorting signal.
The chimeric proteins were stably overexpressed in MDCK cells and visualized by immunofluorescence using a polyclonal antibody to the N-terminus of hGLUT9A. (A) hGLUT9A seen basolaterally (top) (B) hGLUT9A N-terminus spliced onto GLUT1, seen basolaterally (middle) (C) hGLUT9A N-terminus spliced onto GLUT3, seen apically (bottom). D. ATB-BMPA photolabeling of the basolateral surfaces was consistent with the confocal finding. GLUT9a protein was seen on the basolateral surface in Slc2a9A (1) and Slc2a9A/Slc2a1 (2), but not Slc2a9A/Slc2a3 (3). GLUT9 protein is detected as a highly glycosylated set of bands appearing as a smear between 50–75 kDa.