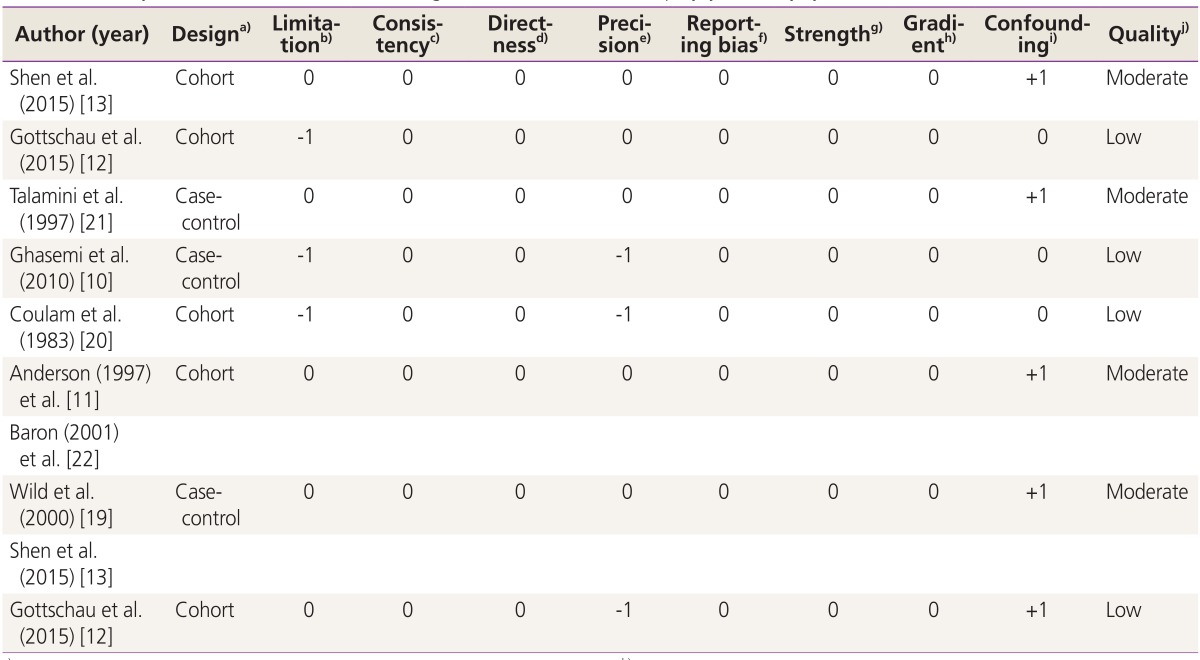
Table 2. Quality assessment of studies addressing the association between polycystic ovary syndrome and breast cancer risk.
a)Randomized trial, cohort study, case-control study, cross-sectional study; b) (a) Incomparability of the groups, (b) different measurement methods, (c) unmatched or unadjusted, (d) short follow-up (in cohort studies), 1 limitation=-1, ≥2 limitation=-2; c)Inverse point estimate compared to overall estimate | no overlap overestimate=-1; d)Interested intervention/exposure, interested outcome, interested population; some (-1) or major (-2) uncertainty about directness; e)If sample size <2,000 and confidence interval includes 1.0=-1, otherwise=0; f)Possibility of selective outcome reporting=-1, otherwise=0; g)RR >2 | <0.5=+1, RR >5 | <0.2)=+2, otherwise=0; h)≥2 levels of exposure=+1, 1 level of exposure=0; i) Adjusted/matched=+1, otherwise=0; j)High: if having no negative score with all positive scores, moderate: if having no negative score with at least one positive score, low: if otherwise.