Figure 6.
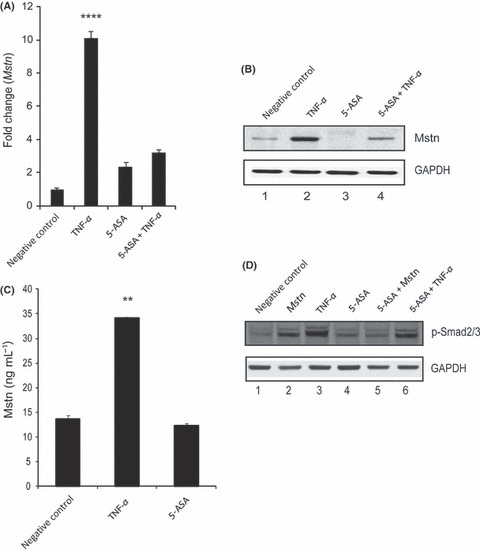
Tumor necrosis factor‐α (TNF‐α) induces myostatin (Mstn) expression, secretion, and signaling in a feed forward mechanism (A) Representative graph showing mRNA expression of Mstn and (B) representative gel showing protein levels of Mstn (∼56 KDa‐full length) in C2C12 cells, untreated (Lane 1), treated with TNF‐α (10 ng mL−1) (Lane 2), 5‐ASA (10 μm mL−1) (Lane 3), and 5‐ASA and TNF‐α (Lane 4) for 72 h in proliferation media. The values are mean ± SE of two independent experiments. Level of significance was compared to the untreated control (****P < 0.0001) (n = 2). GAPDH was used as an internal control for equal protein loading on the gel. (C) Level of secreted Mstn in the cell culture media from C2C12 cells treated with TNF‐α (10 ng mL−1) and 5‐ASA (10 μm mL−1) was determined by EIA. The values are mean ± SE of two independent experiments. The concentration of Mstn is given as ng mL−1, and the level of significance was determined with respect to the negative control (**P < 0.01) (n = 2). (D) Representative gel showing p‐Smad2/3 protein levels in C2C12 cells, untreated (Lane 1), treated with Mstn (3 μg mL−1) (Lane 2), TNF‐α (10 ng mL−1) (Lane 3), 5‐ASA (10 μm mL−1) (Lane 4), 5‐ASA and Mstn (Lane 5), and 5‐ASA and TNF‐α (Lane 6) for 72 h during proliferation. GAPDH was used as an internal control for equal protein loading on the gel.
