Figure 7.
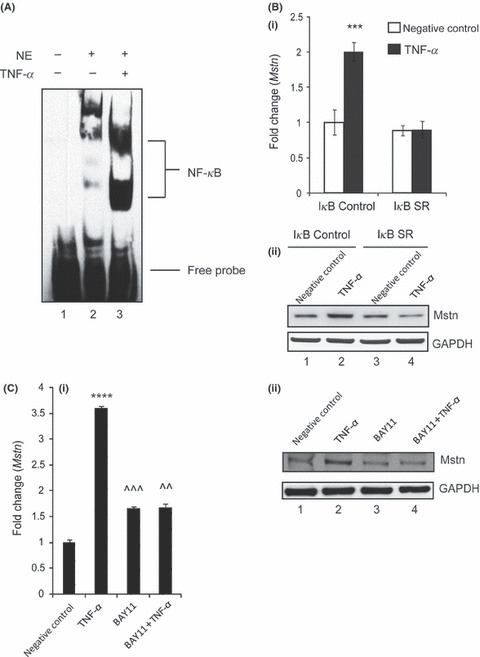
Tumor necrosis factor‐α (TNF‐α) induces myostatin (Mstn) expression via NF‐κB signaling. (A) TNF‐α‐mediated enhancement of NF‐κB binding on mouse Mstn promoter. Electrophoretic mobility shift assay was performed using nuclear extracts from proliferating C2C12 cells treated with TNF‐α (10 ng mL−1) for 72 h. Representative gel showing the significant increase in the binding activity of NF‐κB upon TNF‐α treatment. Lane 1, oligo only; Lane 2, untreated; Lane 3, TNF‐α treated. (B) Representative graph showing mRNA expression of Mstn (i) and representative gel showing protein levels of Mstn (ii) in IκB‐α‐SR‐expressing C2C12 cells treated for 72 h with TNF‐α (10 ng mL−1) in proliferation media; IκB‐α C (control cells) untreated (Lane 1), TNF‐α treated (Lane 2), IκB‐α SR cells untreated (Lane 3), and TNF‐α treated (Lane 4). The values are mean ± SE of two independent experiments. ***P < 0.001 denotes significant increase in mRNA expression when compared to untreated cells (n = 2). GAPDH was used as an internal control for equal protein loading on the gel. (C) Representative graph showing mRNA expression of Mstn (i) and representative gel showing protein levels of Mstn (ii) in C2C12 cells treated for 1 h with BAY11‐7085 (20 μm mL−1) and for 72 h with TNF‐α (10 ng mL−1) in proliferation media; Lane 1, untreated; Lane 2, TNF‐α treated; Lane 3, BAY11‐7085 treated; and Lane 4, BAY11‐7085 and TNF‐α‐treated cells. The values are mean ± SE of two independent experiments. ****P < 0.0001 denotes significant increase in gene expression when compared to untreated cells; ^^P < 0.01 and ^^^P < 0.001 denote significant decrease in mRNA expression when compared to Mstn‐treated cells (n = 2). GAPDH was used as an internal control for equal protein loading on the gel.
