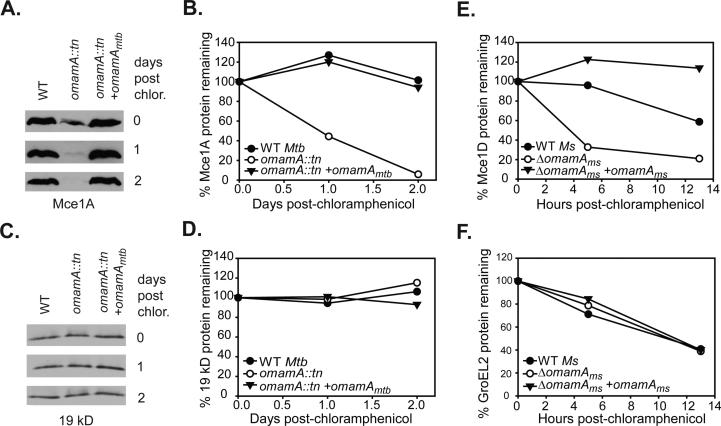
Figure 8.
Mce1 proteins are stabilized by the presence of OmamA. Cultures of M. tuberculosis (Mtb) and M. smegmatis (Ms) were treated with chloramphenicol to prevent further protein synthesis and protein stability was monitored over time by Western blot analysis of whole cell lysates (WCL) with equal protein amount loaded across strains and timepoints. Samples were removed at specific time points, formalin fixed (for M. tuberculosis) and lysed by glass beads to generate WCL. A. The stability of Mce1A in M. tuberculosis strains was followed over a two day time course by Western blot analysis. B. Mce1A protein decay was quantified by measuring band intensity on Western blots using ImageJ. C. The stability of the exported 19 kD lipoprotein in M. tuberculosis over time as monitored by Western blot. D. 19 kD protein abundance was quantitated by ImageJ. Results are representative of at least two independent experiments with M. tuberculosis WT (MBTB178), omamA::tn (MBTB319), and omamA::tn + omamA (MBTB320) strains. E. Decay of Mce1D was quantified in M. smegmatis cultures over 13 hours by measuring band intensity on Western blots using ImageJ. F. Decay of GroEL2 was quantified in M. smegmatis cultures over 13 hours by measuring band intensity on Western blots using ImageJ. Results are representative of at least three independent experiments with M. smegmatis WT +pMV261 (EP1182), ΔomamA +pMV261 (EP1193) and ΔomamA +omamAms (EP1194) strains.