Table 1.
Properties of the solvents used in this study [29].
| Solvents | Molecular structure | Molecular formula | Boiling point (°C) | Acidity (pKa) | Viscosity mPa s (at 20°C) | Surface tension (mN/m) | Solubility parameter (cal1/2 cm−3/2) | Dielectric constant | Electrical conductivity (µs/cm−1) | Molar mass g mol−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Chloroform |
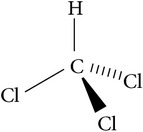
|
CHCl3 | 61 | 15.7 (20 °C) | 0.56 | 27 | 9 | 4.8 | 1 × 10−4 | 119.38 |
| DCM (dichloromethane) |
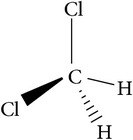
|
CH2Cl2 | 40 | 0.45 | 28 | 10 | 9 | 4 × 10−5 | 84.93 | |
| DMF (dimethylformamide) |
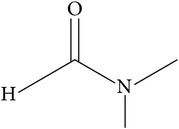
|
C3H7NO | 153 | 0.92 | 35 | 12 | 37 | 6 × 10−2 | 73.09 | |
| TFA (trifluoroacetic acid) |
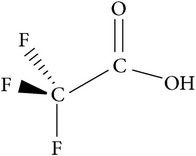
|
C2HF3O2 | 72 | 0.23 | 1.8 | 72.5 | 114.02 |
∗The electric dipole moment is a measure of the separation of positive and negative electrical charges in a system of electric charges, that is, a measure of the charge system's overall polarity.
Surface tension: TFA > DMF > DCM > CHLOROFORM.
Electric conductivity: TFA > DMF > CHLOROFORM > DCM.
Dielectric constant: TFA > DMF > DCM > CHLOROFORM.
Boiling point (°C): DMF > TFA > CHLOROFORM > DCM.
