FIGURE 5.
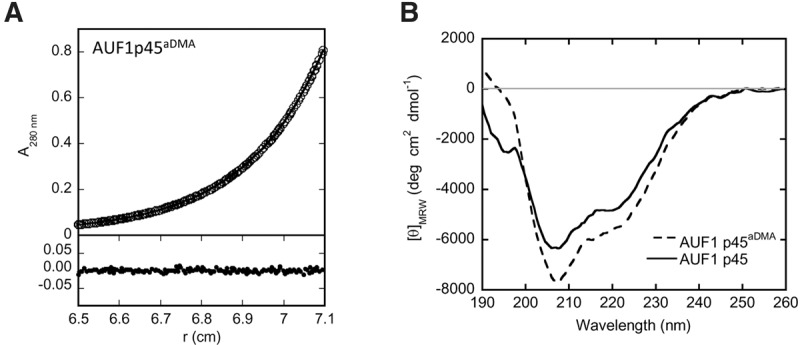
Features of AUF1 p45 and AUF1 p45aDMA. (A) In sedimentation equilibrium experiments (analytical ultracentrifugation), AUF1 p45aDMA (5 µM of protein) was analyzed at 14,000 rpm at 20°C using the software provided by Beckman Coulter. The upper panel shows the experimental data (circles) and the fit (line), and the lower panel shows the deviation of the experimental data and the fit. After reaching equilibrium, the molecular mass of AUF1 p45aDMA was determined to be 42.1 kDa (theoretical MW of the protein monomer = 38.3 kDa). No aggregation of AUF1 p45aDMA was observed in the course of the experiment. (B) Far-UV circular dichroism (CD) spectra of AUF1 p45 and AUF1 p45aDMA were recorded. The acquired data were normalized to mean residue weight (MRW) ellipticities.
