Figure 2.
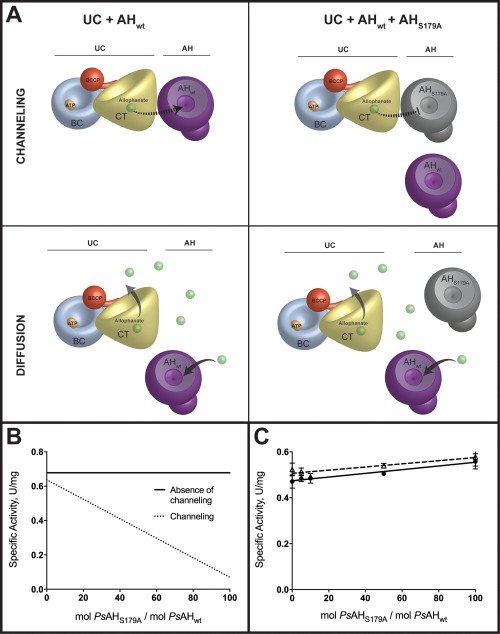
Alophanate is not channeled from PsUC to PsAH. (A) A model for the channeling or diffusion of allophanate from UC to AH as assayed by the method of Geck and Kirsch.14 The PsAHS179A inactivated enzyme interferes with substrate channeling by displacing PsAHwt and alters the overall rate. The PsAHS179A inactivated enzyme does not alter the reaction kinetics if the allophanate intermediate freely diffuses from PsUC to PsAH. The cartoon representations of UC and AH are based on published crystal structures (4GYR, 4IST, and 3VA7) with the biotin carboxylase (BC; blue), carboxyltransferase (CT; yellow), biotin carboxyl carrier protein (BCCP; red) and allophanate hydrolase (AH; purple) domains/subunits illustrated to an approximate of their relative scale. (B) Predicted plots of specific activity as a function of increasing ratios of inactivated (PsAHS179A) enzyme to wild‐type enzyme. The total enzyme concentration (PsAH179A + PsAHwt) remains constant for all ratios (C) The specific activities for the complete conversion of , MgATP and urea to NH3 and CO2 were measured at increasing ratios of inactive PsAHS179A to PsAHwt in the absence (closed circles; solid line) and presence (open triangles; dashed line) of 22% PEG4K. The total enzyme concentration (PsAH179A + PsAHwt) was kept constant at all ratios.
