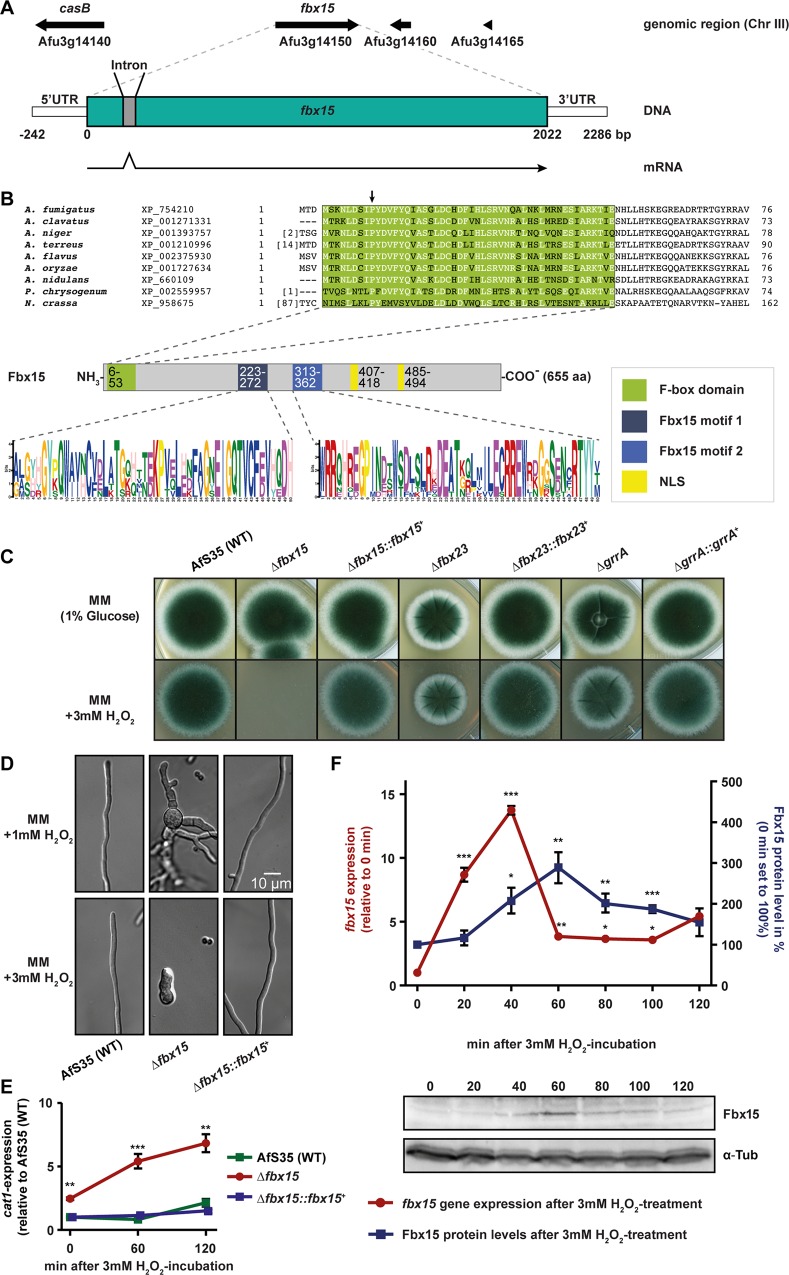
Fig 1. Aspergillus fumigatus F-box domain protein Fbx15 is indispensible for oxidative stress response.
(A) fbx15 genomic locus, structure and transcripts. (B) The primary sequence of A. fumigatus Fbx15 F-box domain was compared with fungal homologues by ClustalW alignment. Highly conserved amino acids are marked in white; an arrow indicates the characteristic proline residue at position seven of the F-box domain. (C) fbx deletion mutants Δfbx15 and to less extent Δfbx23 and ΔgrrA showed increased oxidative stress sensitivity, which could be complemented by reintroduction of the wild type fbx-genes. (D) Δfbx15 mutant showed hyphal defects at low H2O2 concentrations. (E) Expression levels of cat1 encoding a mycelial catalase were increased in the Δfbx15 mutant, whereas wild type and complemented strain showed only slightly elevated cat1 mRNA levels after H2O2 exposure. Mean values ± s.d. of N = 3 independent experiments are shown. P-values were calculated using two-sample t-test (**P<0.01; ***P<0.001). (F) fbx15 transcript expression in AfS35 (WT) showed a rapid increase in the first 20 min upon oxidative stress and with a short delay increased protein levels, which are detected by an Fbx15 specific antibody. Mean values ± s.d. of N = 3 independent experiments are shown. P-values were calculated using two-sample t-test (*P<0.05; **P<0.01; ***P<0.001).