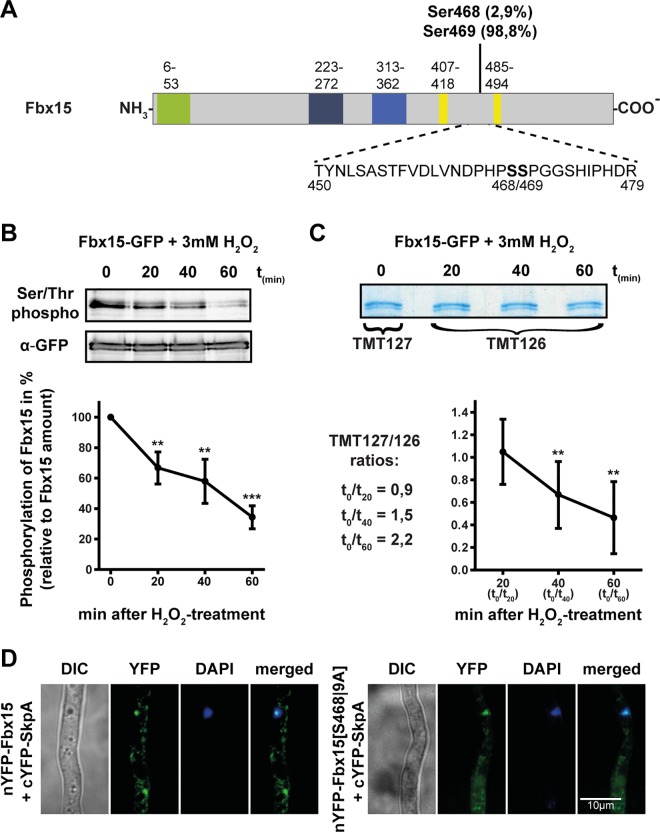
Fig 2. Fbx15 phosphorylation decreases during oxidative stress treatment and shifts the cellular interaction site with SkpA to the nucleus.
(A) Phosphorylated peptide of Fbx15 identified by LC-MS/MS after TAP-purification. Phosphorylation probabilities are based on NetPhos 2.0. (B) Immunoblotting of purified Fbx15-GFP before and after 3 mM H2O2 treatment with an S/T-phospho-specific antibody revealed rapid dephosphorylation of Fbx15. The graph represents mean values ± s.d. of N = 3 independent experiments, with S/T-phospho-specific signal intensities normalized to total Fbx15 amount detected by GFP-specific antibody. Normalized Signal intensities were analyzed using two-sample t-test (**P<0.01; ***P<0.001). (C) Peptides of purified Fbx15-GFP before and after incubation with 3 mM H2O2 were differentially labeled with TMT isobaric mass labeling reagent. LC-MS/MS determined ratios of the TMT reporter-ion intensities for the MS2-spectra of the phosphopeptide are given. The graph on the right represents the reciprocal values, which confirmed specific dephosphorylation of S468/469 upon oxidative stress. Ratios of the TMT reporter-ion intensities from normalized phosphopeptides of N = 2 independent experiments were analyzed by one-way ANOVA followed by Tukey’s HSD test (**P<0.01). (D) BiFC signals of Fbx15 and SkpA occurred mostly in the cytoplasm, whereas unphosphorylated Fbx15[S468|9A] interacted with SkpA primarily in the nucleus.