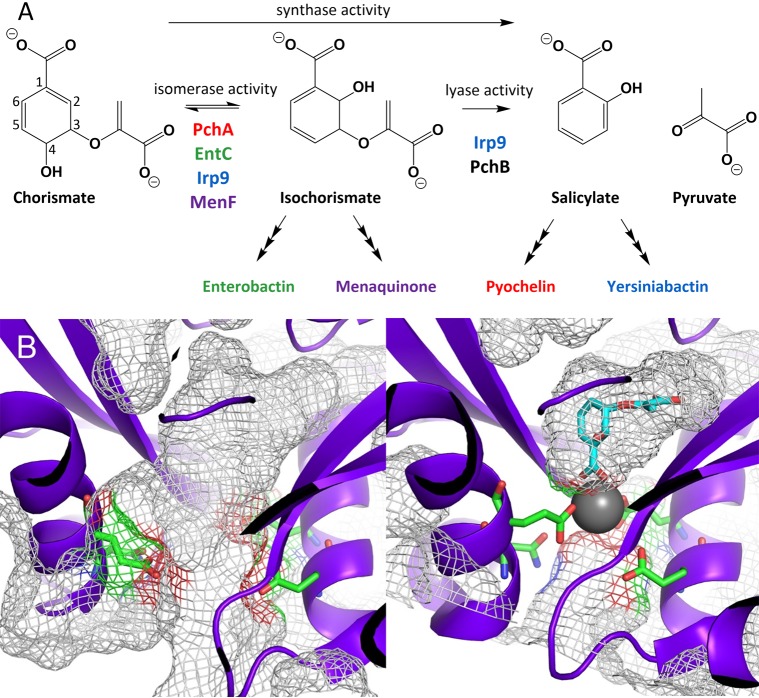
Figure 1.
Reactions and representative conformational states of MST enzymes. (A) MST enzymes that isomerize chorismate to isochorismate (isomerase activity) and enzymes that eliminate the pyruvyl enol substituent (lyase activity). Synthase activity refers to enzymes that perform isomerase activity and subsequently perform lyase activity. The color scheme introduced here will be maintained throughout: PchA = red; EntC = green; Irp9 = blue; MenF = purple. PchB (black) is not an MST enzyme; however, PchB performs the lyase reaction and is used in this work to generate the fluorescent salicylate for isomerase-only enzymes. (B) Open (right, PDB ID 3BZM) and closed (left, PDB ID 3BZN) forms of MenF. The closed form has a magnesium ion (gray sphere), but the isochorismate (cyan sticks) was modeled by superposition of the EntC structure (PDB ID 3HWO). In both structures, the amino acids that serve as metal ligands are depicted as green sticks. It should be noted that in the closed structure, the passageway between the active site and the solvent is occluded by the presence of the magnesium ion. In other words, there is no portal for substrate or product entry or egress without dissociation of the catalytic magnesium. The gray mesh shown here represents the surface topology, including cavities, pockets, and voids, as calculated by CASTp.