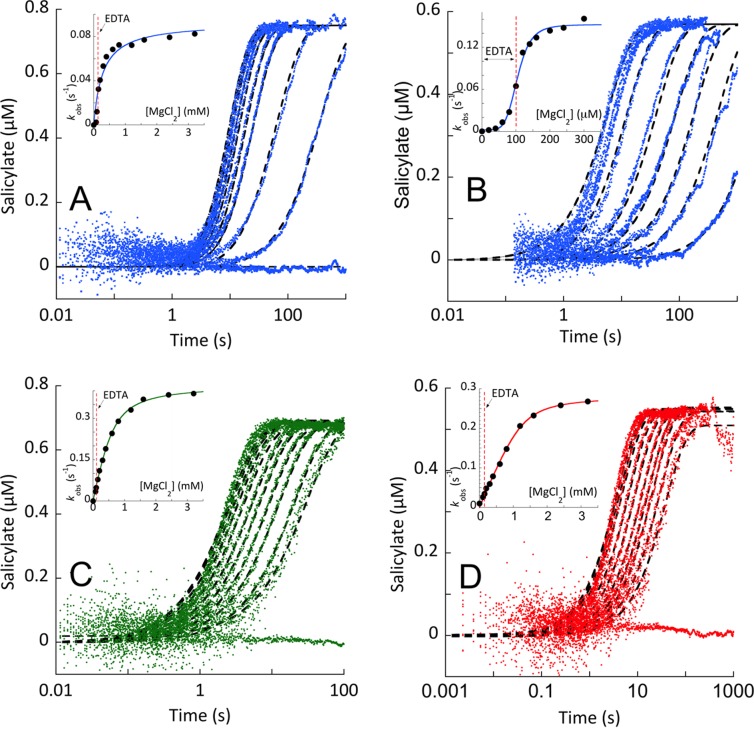
Figure 7.
Single-turnover reactions of Irp9, EntC, and PchA. Single-turnover conditions were established on the basis of the Kd values for substrates obtained by titration of each enzyme’s intrinsic fluorescence (Figure 6). (A, C, D) Chorismate or (B) isochorismate was added to an enzyme concentration sufficient to provide greater than 90% substrate bound. This complex was prepared in a buffer containing EDTA (100 μM final concentration after double mix) to ensure that no turnover occurred prior to mixing with magnesium ions. The E·S complex was then mixed with pseudo-first-order concentrations of Mg(II). The Irp9 reactions are shown in (A) chorismate and (B) isochorismate. The (C) EntC and (D) PchA reactions with chorismate included excess PchB in the second mix. These conditions approximate first-order conditions under the assumption that the release of Mg(II) and products are fast relative to reversible catalytic steps (Figure 6D). The data were fit to single-exponential events (eq 4), and the dependence of the observed rate constant is shown in the inset of each plot.