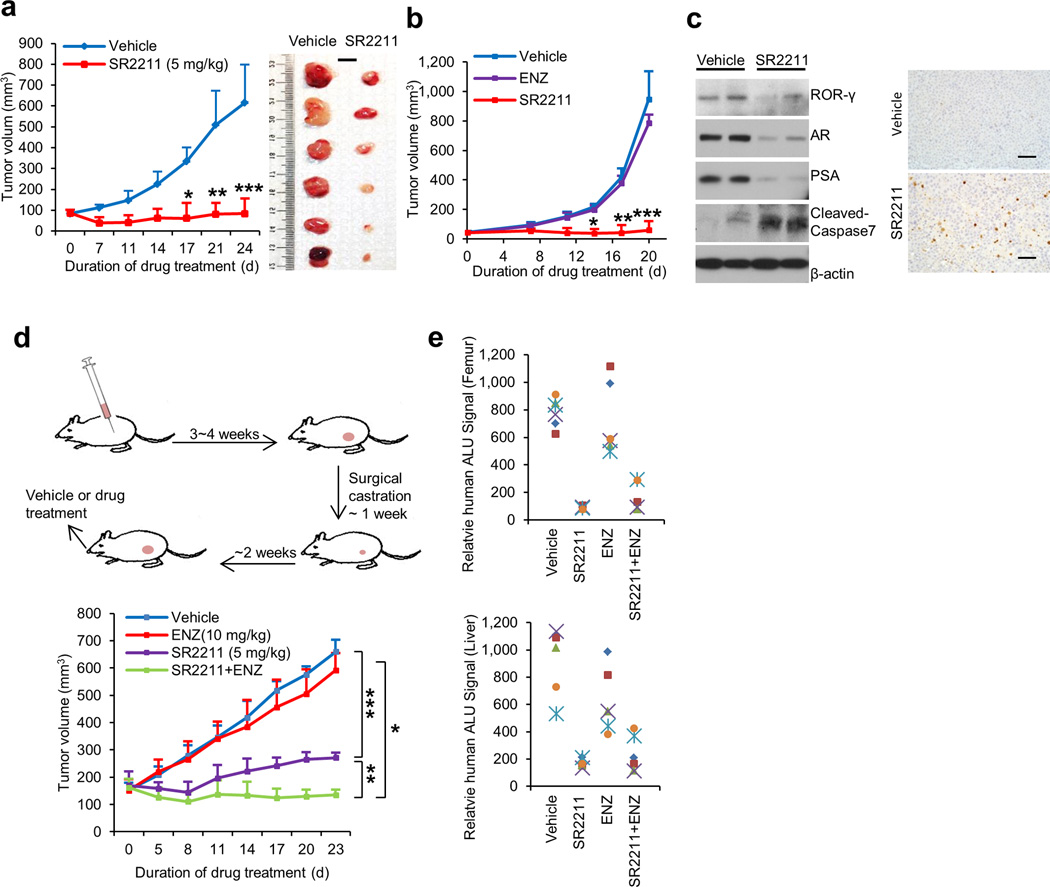
Figure 6. ROR-γ antagonists potently inhibit tumor growth and metastasis.
(a) and (b) Effects of indicated treatments (SR2211, 5 mg/kg, i.p., 5 times a week; ENZ, 10 mg/kg, oral, 5 times a week; or vehicle) on growth of C4-2B or 22Rv1 xenografts (n = 6 mice per group). Treatment started when C4-2B tumors reached approximately100 mm3, or 22Rv1 tumors reached approximately 50 mm3. Mean tumor volume ± s.e.m and representative tumor images are shown. Scale bar is 1cm. Significance was calculated using Student’s t-test with the following p values: for C4-2B exnografts, * p = 0.000293, ** p = 6.67E-05, *** p=1.13E-05; for 22Rv1 xenografts, # p = 3.03E-08, ** p = 1.77E-09, *** p = 1.14E-08.
(c) Immunoblotting (left) of C4-2B xenograft tumors after 24 days of treatment with vehicle or SR2211 as in (a). Cell lysate for each lane was from homogenized tumor tissues randomly combined from three mice of vehicle or SR2211 group. Anti-cleaved caspase-3 IHC images of tumor sections were shown at right. Scale bar is 50 µm.
(d) Schematics illustrating CRPC-VCaP xenograft tumor establishment and treatment (left). Castrated mice bearing the CRPC-VCaP xenografts (n = 6 mice per group) received vehicle, SR2211 (i.p.; 5 mg/kg), ENZ (oral; 10 mg/kg), or a combination of SR2211 and ENZ, as indicated, 5 times a week. Mean tumor volume ± s.e.m was shown. Significance was calculated using Student’s t-test. * p = 1.98E-10, ** p = 1.46E-10, *** p = 7.21E-13.
(e) Mice bearing VCaP xenografts as in (d) treated with vehicle, SR2211, ENZ or combination for 23 days were assessed for spontaneous metastasis to the femur (bone marrow) and liver. Genomic DNA isolated from these sites was analyzed for metastasized cells by measuring human Alu sequence (by Alu-qPCR).