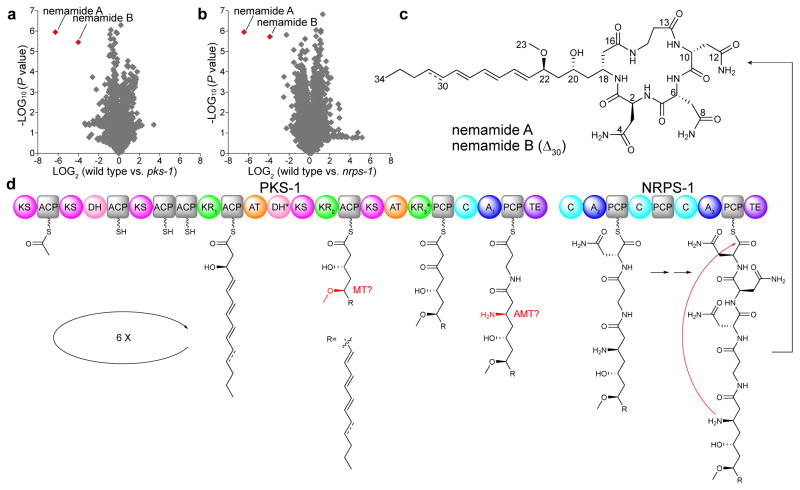
Figure 1. Discovery and biosynthesis of the nemamides.
(a,b) Comparison of average peak areas for metabolite features in wild-type worms versus pks-1 mutant worms (a) and in wild-type worms versus nrps-1 mutant worms (b), with nemamide A and B highlighted in red. In a and b, extracts from three separate cultures were analyzed for each strain, and P values were calculated in XCMS using a Welch’s t-test. (c) Chemical structures of nemamide A and B. (d) Proposed biosynthetic assembly line for the nemamides. Domain abbreviations: acyl transferase (AT), acyl carrier protein (ACP), ketosynthase (KS), ketoreductase (KR), dehydratase (DH), methyltransferase (MT), aminotransferase (AMT), adenylation (A), peptidyl carrier protein (PCP), condensation (C), and thioesterase (TE). Domains labeled with an asterisk are predicted to be inactive based on the nemamide structures. The KR and A domains are labeled with numbers for further discussion in Supplementary Figure 13 and Supplementary Table 4. KR1 is predicted to be B-type, while KR2 and KR3 are neither A- nor B-type (Supplementary Fig. 13). Given that the nemamides contain four amino acids and that PKS-1 and NRPS-1 have only three A domains, A2 may act twice to incorporate two Asn residues.