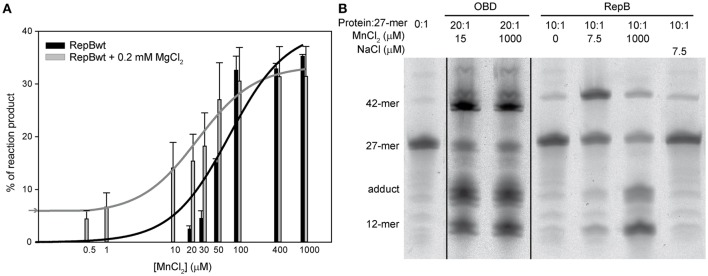
Figure 8.
Nicking and strand-transfer activity of RepB6 on ssDNA oligos in the presence of different metal cation concentrations. (A) The vertical bar graph shows the percentage of reaction products rendered by RepB6, in the presence or in the absence of 0.2 mM of MgCl2, when the indicated concentrations of MnCl2 were added. The 27-mer oligo substrate (500 nM), labeled in 3′ with the fluorescent dye Cy5, and a 10-fold molar excess of the unlabeled 30-mer oligo were incubated with the protein at 37°C for 1 min. The assays were performed at a protein:oligo substrate molar ratio of 1:10. Vertical bars represent the average value of three different measurements and error bars are standard deviations. The activity curves of RepB6 supplemented or not with MgCl2 were fitted by nonlinear regression (solid lines) to a ligand binding model assuming a single class of binding site for Mn2+. The best fit values for the apparent dissociation constant for RepB6 was 24.1 ± 6.9 μM in the presence of 0.2 mM of MgCl2, and 80.5 ± 26.2 μM in the absence of MgCl2. The percentage of reaction products measured in the presence of 0.2 mM of MgCl2 with no MnCl2 added ( ) is indicated on the y-axis. (B) Reaction product pattern generated by the nicking and strand-transfer activities of RepB6 and OBD on ssDNA oligos. The assays were performed as those depicted in (A), at the protein:oligo substrate molar ratio and MnCl2 concentration indicated on the top of each lane. As a control, the reaction was also carried out with NaCl instead of manganese salt. The resultant fluorescent oligos were analyzed, visualized and quantified as in Figure 7. To compare the reactions products generated by the activity of OBD and RepB6 the images from different gels acquired and processed under the same conditions have been grouped and indicated by dividing lines.
) is indicated on the y-axis. (B) Reaction product pattern generated by the nicking and strand-transfer activities of RepB6 and OBD on ssDNA oligos. The assays were performed as those depicted in (A), at the protein:oligo substrate molar ratio and MnCl2 concentration indicated on the top of each lane. As a control, the reaction was also carried out with NaCl instead of manganese salt. The resultant fluorescent oligos were analyzed, visualized and quantified as in Figure 7. To compare the reactions products generated by the activity of OBD and RepB6 the images from different gels acquired and processed under the same conditions have been grouped and indicated by dividing lines.