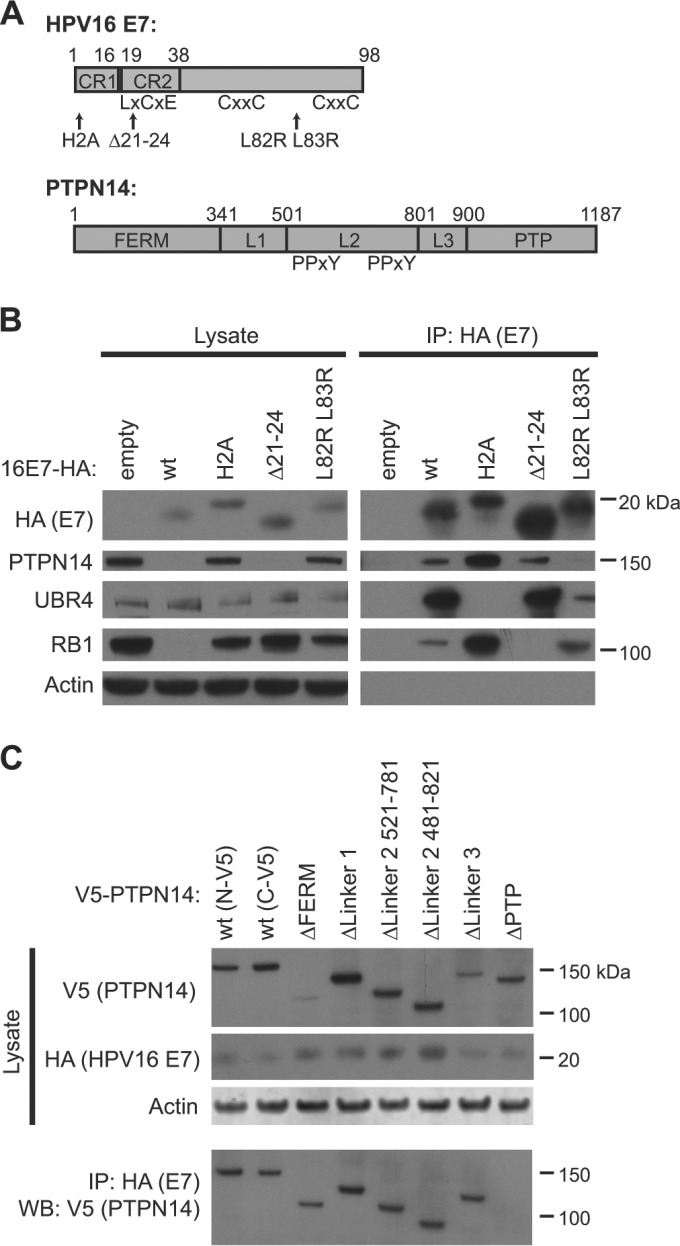
FIG 3 .
Identification of protein binding sites on HPV16 E7 and on PTPN14. (A) Schematic of HPV16 and PTPN14 domain organization and mutants used in the study (39). (B) N/Tert-1 cells expressing wild-type (wt) HPV16 E7-Flag-HA, one of several mutants of HPV16 (E7), or the empty vector were subjected to immunoprecipitation with HA antibody. Immunoprecipitates were separated by SDS-PAGE and Western blotted using antibodies to HA, PTPN14, UBR4, RB1, and actin. (Left panels) Protein levels in input lysates. (Right panels) Proteins recovered after immunoprecipitation with anti-HA antibody. (C) N/Tert-1 cells expressing wild-type HPV16 E7-Flag-HA were transduced with retroviruses expressing V5-tagged PTPN14 or one of several deletion mutant forms of PTPN14 and were harvested 48 h posttransduction. Cell lysates were subjected to immunoprecipitation with HA antibody. Immunoprecipitates were separated by SDS-PAGE and Western blotted using antibodies to HA, V5, and actin. (Top panels) Protein levels in input lysates. (Bottom panel) V5-tagged PTPN14 recovered after immunoprecipitation with anti-HA antibody.