Abstract
AIMS--To adapt the polymerase chain reaction (PCR) technique of HIV detection to paraffin wax embedded brain tissue and to compare the results with those obtained using frozen tissue. METHODS--HIV antigen and HIV proviral DNA were detected in specimens of frontal lobe using immunohistochemistry and PCR, respectively. DNA was extracted from fresh tissue using standard methods whereas the technique for extracting DNA from paraffin wax embedded tissue was partly modified. RESULTS--Twenty cases were examined. HIV DNA was detected in 16 cases in frozen specimens. Of these, 15 were also positive when paraffin wax embedded material was analysed. CONCLUSIONS--This study shows that HIV proviral DNA can be detected in formalin fixed, paraffin wax embedded brain tissue by PCR. The results obtained from paraffin wax embedded specimens showed a similar degree of reliability to those from fresh frozen brain. Factors such as fixative, fixation time, and delay in performing post mortem examinations did not seem to influence PCR amplification as positive results were obtained with specimens left in fixative for up to eight months, as well as in cases where post mortem examinations had been delayed for up to four days.
Full text
PDF
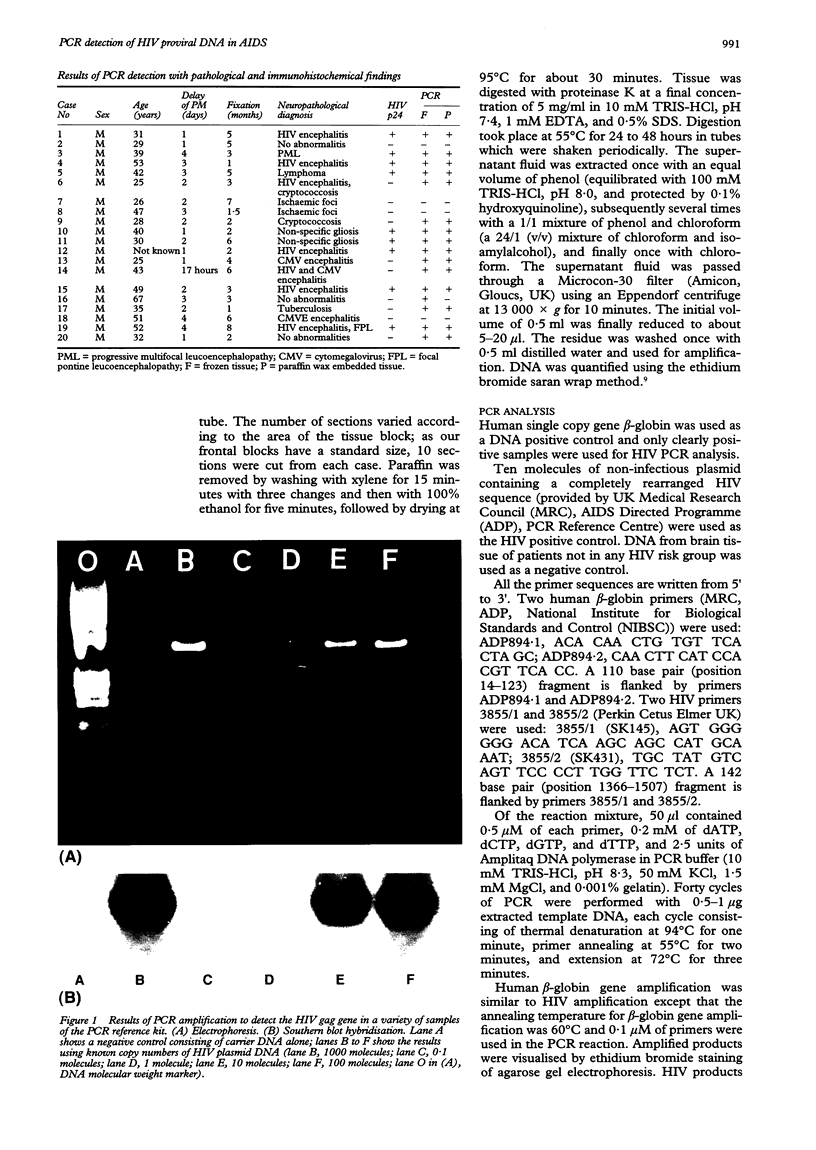



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An S. F., Fleming K. A. Removal of inhibitor(s) of the polymerase chain reaction from formalin fixed, paraffin wax embedded tissues. J Clin Pathol. 1991 Nov;44(11):924–927. doi: 10.1136/jcp.44.11.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell N. H., Burns B. F. The effects of fixative type and fixation time on the quantity and quality of extractable DNA for hybridization studies on lymphoid tissue. Exp Hematol. 1988 Sep;16(8):730–732. [PubMed] [Google Scholar]
- Brandwein M. S., Nuovo G. J., Biller H. Analysis of prevalence of human papillomavirus in laryngeal carcinomas. Study of 40 cases using polymerase chain reaction and consensus primers. Ann Otol Rhinol Laryngol. 1993 Apr;102(4 Pt 1):309–313. doi: 10.1177/000348949310200411. [DOI] [PubMed] [Google Scholar]
- Bresters D., Cuypers H. T., Reesink H. W., Chamuleau R. A., Schipper M. E., Boeser-Nunnink B. D., Lelie P. N., Jansen P. L. Detection of hepatitis C viral RNA sequences in fresh and paraffin-embedded liver biopsy specimens of non-A, non-B hepatitis patients. J Hepatol. 1992 Jul;15(3):391–395. doi: 10.1016/0168-8278(92)90075-z. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Viscidi R. P., Saint-Antoine P., Kessous A., Yolken R. H. The polymerase chain reaction: a new tool for the understanding and diagnosis of HIV-1 infection at the molecular level. Mol Cell Probes. 1991 Aug;5(4):241–259. doi: 10.1016/0890-8508(91)90046-m. [DOI] [PubMed] [Google Scholar]
- Kellokoski J. K., Syrjänen S. M., Chang F., Yliskoski M., Syrjänen K. J. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV infections. J Oral Pathol Med. 1992 Nov;21(10):459–464. doi: 10.1111/j.1600-0714.1992.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Lager D. J., Burgart L. J., Slagel D. D. Epstein-Barr virus detection in sequential biopsies from patients with a posttransplant lymphoproliferative disorder. Mod Pathol. 1993 Jan;6(1):42–47. [PubMed] [Google Scholar]
- Lai-Goldman M., Lai E., Grody W. W. Detection of human immunodeficiency virus (HIV) infection in formalin-fixed, paraffin-embedded tissues by DNA amplification. Nucleic Acids Res. 1988 Aug 25;16(16):8191–8191. doi: 10.1093/nar/16.16.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman W. D., Kress Y., Kure K., Rashbaum W. K., Rubinstein A., Soeiro R. Detection of HIV in fetal central nervous system tissue. AIDS. 1990 Sep;4(9):917–920. doi: 10.1097/00002030-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Morgello S. Epstein-Barr and human immunodeficiency viruses in acquired immunodeficiency syndrome-related primary central nervous system lymphoma. Am J Pathol. 1992 Aug;141(2):441–450. [PMC free article] [PubMed] [Google Scholar]
- Morshed S. A., Nishioka M., Saito I., Komiyama K., Moro I. Increased expression of Epstein-Barr virus in primary biliary cirrhosis patients. Gastroenterol Jpn. 1992 Dec;27(6):751–758. doi: 10.1007/BF02806528. [DOI] [PubMed] [Google Scholar]
- Nawa A., Nishiyama Y., Kikkawa F., Kawai M., Mano H., Goto S., Suganuma N., Tomoda Y., Nakashima N. Detection of human papillomaviruses from histologically normal lymph nodes of Japanese cervical cancer patients by nested polymerase chain-reaction assay. Int J Cancer. 1993 Apr 1;53(6):932–937. doi: 10.1002/ijc.2910530611. [DOI] [PubMed] [Google Scholar]
- Nicoll J. A., Kinrade E., Love S. PCR-mediated search for herpes simplex virus DNA in sections of brain from patients with multiple sclerosis and other neurological disorders. J Neurol Sci. 1992 Dec;113(2):144–151. doi: 10.1016/0022-510x(92)90242-d. [DOI] [PubMed] [Google Scholar]
- Niedermeyer H., Fellbaum C., Hansmann M. L., Kraus I., Alavaikko M. J., Busch R., Pütz B., Fischer R., Höfler H. Einfluss von Epstein-Barr Virusgenom auf die Uberlebenszeit von Patienten mit M. Hodgkin. Verh Dtsch Ges Pathol. 1992;76:173–176. [PubMed] [Google Scholar]
- Ohta M., Ikeda M. [The significance of HPV 16, 18 infection and the DNA ploidy associated with the progression of uterine cervical dysplasia]. Nihon Sanka Fujinka Gakkai Zasshi. 1993 Jun;45(6):540–546. [PubMed] [Google Scholar]
- Pang S., Koyanagi Y., Miles S., Wiley C., Vinters H. V., Chen I. S. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990 Jan 4;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Saltzstein D. R., Orihuela E., Kocurek J. N., Payne D. A., Chan T. S., Tyring S. K. Failure of the polymerase chain reaction (PCR) to detect human papilloma virus (HPV) in transitional cell carcinoma of the bladder. Anticancer Res. 1993 Mar-Apr;13(2):423–425. [PubMed] [Google Scholar]
- Saluz H., Jost J. P. A simple high-resolution procedure to study DNA methylation and in vivo DNA-protein interactions on a single-copy gene level in higher eukaryotes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2602–2606. doi: 10.1073/pnas.86.8.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair E., Gray F., Scaravilli F. PCR detection of HIV proviral DNA in the brain of an asymptomatic HIV-positive patient. J Neurol. 1992 Oct;239(8):469–470. doi: 10.1007/BF00856814. [DOI] [PubMed] [Google Scholar]
- Sinclair E., Scaravilli F. Detection of HIV proviral DNA in cortex and white matter of AIDS brains by non-isotopic polymerase chain reaction: correlation with diffuse poliodystrophy. AIDS. 1992 Sep;6(9):925–932. doi: 10.1097/00002030-199209000-00003. [DOI] [PubMed] [Google Scholar]
- Tokuda Y., Nakamura T., Satonaka K., Maeda S., Doi K., Baba S., Sugiyama T. Fundamental study on the mechanism of DNA degradation in tissues fixed in formaldehyde. J Clin Pathol. 1990 Sep;43(9):748–751. doi: 10.1136/jcp.43.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux R., Lacroix-Ciaudo C., Blanche S., Cumont M. C., Henin D., Gray F., Boccon-Gibod L., Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992 Jan;140(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Vesy C. J., Greenson J. K., Papp A. C., Snyder P. J., Qualman S. J., Prior T. W. Evaluation of celiac disease biopsies for adenovirus 12 DNA using a multiplex polymerase chain reaction. Mod Pathol. 1993 Jan;6(1):61–64. [PubMed] [Google Scholar]
- Wiestler O. D., Leib S. L., Brüstle O., Spiegel H., Kleihues P. Neuropathology and pathogenesis of HIV encephalopathies. Acta Histochem Suppl. 1992;42:107–114. [PubMed] [Google Scholar]
- Woodall C. J., Watt N. J., Clements G. B. Simple technique for detecting RNA viruses by PCR in single sections of wax embedded tissue. J Clin Pathol. 1993 Mar;46(3):276–277. doi: 10.1136/jcp.46.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ben-Ezra J., Winberg C., Colombero A. M., Rappaport H. Analysis of antigen receptor gene rearrangements in ethanol and formaldehyde-fixed, paraffin-embedded specimens. Lab Invest. 1990 Jul;63(1):107–114. [PubMed] [Google Scholar]
- de Franchis R., Cross N. C., Foulkes N. S., Cox T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 1988 Nov 11;16(21):10355–10355. doi: 10.1093/nar/16.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bommel P. F., van den Brule A. J., Helmerhorst T. J., Gallee M. P., Gaarenstroom K. N., Walboomers J. M., Meijer C. J., Kenemans P. HPV DNA presence and HPV genotypes as prognostic factors in low-stage squamous cell cervical cancer. Gynecol Oncol. 1993 Mar;48(3):333–337. doi: 10.1006/gyno.1993.1058. [DOI] [PubMed] [Google Scholar]