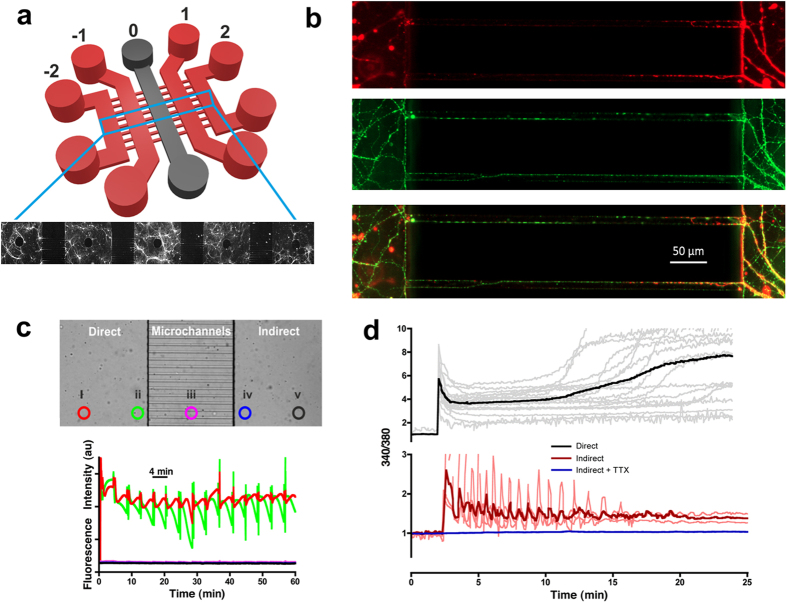
Figure 1. Functional synaptic communication between environmentally-isolated neuronal networks.
(a) Schematic of the microfluidic device showing the five parallel culture chambers (−2, −1, 0, 1, 2). (b) Hippocampal neurons cultured in microfluidic devices labeled to distinguish dendrites (MAP2, red) and axons (tubulin, green). Scale bar = 50 μm. (c) Validation of microfluidic protocol for insult delivery. (Top) Light microscopy image of the microchannel barrier separating the insulted chamber (‘direct’) from the insult-free chamber (‘indirect’). Highlighted circles represent the points of fluorescein fluorescence monitoring. (Bottom) Fluorescent intensity profiles obtained from the five regions of interest (i–v) using the insult protocol detailed in Materials and Methods. No cross contamination of fluorescein was observed in any indirect chambers, whilst an almost constant fluorescence profile was achieved across the width of the insulted chamber (circle i). (d) A direct excitotoxic challenge (100 GG) leads to delayed Ca2+ deregulation (n = 4) and downstream (indirect), activity-dependent (+TTX, blue traces, n = 3) Ca2+ spiking activity (red traces, n = 3). Traces are representative of individual neuronal responses (light color) and the mean for all recorded cells (dark color). Experiments were performed using independent neuronal preparations.