Abstract
Raynaud’s phenomenon is frequently observed in systemic sclerosis (SSc) patients, and cold- or stress-induced norepinephrine (NE) has been speculated to be associated with vasoconstriction. Objective was to elucidate the role of NE in fibrosis in SSc. IL-6 is a potent stimulator of collagen production in fibroblasts. NE enhanced IL-6 production and proliferation more significantly in SSc fibroblasts than in normal fibroblasts. Furthermore, the production of IL-6 and phosphorylation of p38 in SSc fibroblasts was enhanced by adrenergic receptor (AR)β agonist, isoproterenol, but not ARα agonist, oxymetazoline. ARβ blocker, propranolol, inhibited NE-induced IL-6 production and phosphorylation of p38 in SSc fibroblasts. NE-induced IL-6 was significantly inhibited by p38 inhibitor, SB203580, suggesting that NE-induced phosphorylation of p38 via ARβ enhances IL-6 production in SSc fibroblasts. NE-induced phosphorylation of ERK1/2 via ARα inhibited IL-6 production in SSc fibroblasts. Combined treatment with NE and endothelin-1 resulted in an additive increase in IL-6 production in SSc fibroblasts. NE-induced IL-6/IL-6 receptor trans-signaling increased the production of collagen type I in SSc fibroblasts, and both propranolol and SB203580 inhibited NE-induced collagen production. These results suggest that cold exposure and/or emotional stress-induced NE might contribute to the skin fibrosis via potentiation of IL-6 production from fibroblasts in SSc.
Norepinephrine (NE) is primarily released from the postganglionic neurons of the sympathetic nervous system as a neurotransmitter. NE is also synthesized in the locus coeruleus and adrenal medulla. It is well known that emotional stress and cold stimulation increase the systemic and/or local NE levels1,2,3. NE binds to the adrenergic receptor (AR) in multiple organs, including the heart, lungs, brain and skin. In the skin, ARβ2 is primarily expressed on the surface of keratinocytes, dermal fibroblasts and melanocytes4,5,6. Activation of ARβ2 signaling impairs re-epithelialization, resulting in delayed wound healing in both human and murine skin7,8. In in vitro studies, activation of ARβ2 by isoproterenol enhances migration and alters both the actin cytoskeleton and focal adhesion distribution in dermal fibroblasts, suggesting that NE:AR signaling regulates the function of dermal fibroblasts7,8,9.
Systemic sclerosis (SSc) is a connective tissue disorder characterized by the development of fibrosis in the skin and internal organs as well as microvascular dysfunction. Raynaud’s phenomenon is commonly observed in patients with SSc and characterized by the presence of episodic vasospasms and ischemia of the extremities in response to cold or emotional stress. It has also been speculated that cold- or stress-induced NE stimulates ARα on pericytes and/or vascular smooth muscle cells, thus resulting in vasoconstriction10,11. In addition, SSc patients treated with the ARα2c antagonist exhibit improvements in the symptoms of Raynaud’s phenomenon induced by cold stimulation12, suggesting that NE is involved in the pathogenesis of vasculopathy in SSc. However, the roles of NE in the development of skin fibrosis associated with SSc are not well investigated.
IL-6 is a pleiotropic multifunctional cytokine produced by various cells, such as lymphocytes, monocytes and fibroblasts13. IL-6 has various immunological functions, for example, it induces B cell differentiation to produce immunoglobulin, stimulates Th17 differentiation in the presence of transforming growth factor (TGF)-β and inhibits the induction of TGFβ-induced regulatory T cells14,15. In addition, IL-6 is considered to be involved in the pathogenesis of several autoimmune diseases, including SSc and rheumatoid arthritis16. With respect to IL-6 and SSc, many in vivo and in vitro studies have shown that IL-6 plays an important role in the pathogenesis of fibrosis in SSc. For example, the serum IL-6 levels are significantly elevated in SSc patients of early stage17,18 and correlate with the total skin thickness score in persons with this disease19. Moreover, a prominent expression of IL-6 is observed in dermal fibroblasts, mononuclear cells and endothelial cells in the patient’s skin of early stage of diffuse cutaneous type (dc)SSc18,20, suggesting that dermal fibroblasts are an important source of IL-6 in affected skin lesions. In in vitro studies, skin dermal fibroblasts derived from SSc patients have been found to produce high levels of IL-6, and the complex of IL-6 and soluble IL-6 receptor (sIL-6R) has been shown to stimulate SSc fibroblasts via gp130 to differentiate and proliferate, resulting in collagen overproduction and fibrosis18,21,22,23. In addition, several case studies have reported softening of the skin in SSc patients after the treatment with an anti-IL-6 receptor antibody (tocilizumab), supporting the essential role of IL-6 in the pathogenesis of skin fibrosis associated with SSc24. Recently, phase II clinical trial on tocilizumab in SSc patients revealed that the reduction of skin sclerosis in tocilizumab group tended to be greater than those in placebo group25. It has also been reported that IL-1α, platelet-derived growth factor (PDGF), tumor necrosis factor-α (TNF-α) and CD154/CD40 interactions induce IL-6 production in fibroblasts22,26,27,28. However, the precise mechanisms underlying the onset of IL-6-induced fibrosis in the setting of SSc and whether NE stimulation enhances IL-6 production in SSc fibroblasts remain unclear. In addition, the relationship between NE and endothelin-1 (ET-1), which is associated with skin fibrosis in SSc patients, in the pathogenesis of skin sclerosis of SSc is unknown. In this study, we examined the mechanisms of NE-induced IL-6 production in SSc fibroblasts and aimed to clarify the roles of NE in the pathogenesis of fibrosis in SSc.
Results
NE-induced IL-6 production was significantly higher in the SSc fibroblasts than in the normal fibroblasts
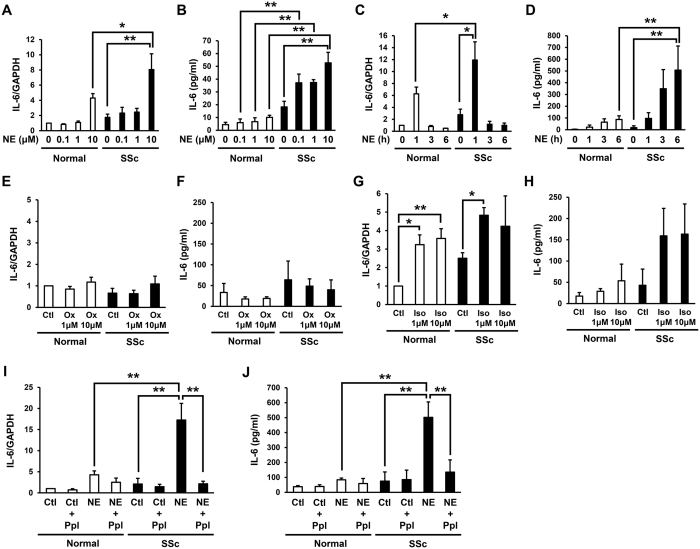
In order to assess the effects of NE on IL-6 production from normal and SSc dermal fibroblasts, the secreted protein and mRNA levels of IL-6 in normal and SSc fibroblasts treated with NE were examined. The mRNA and secreted protein levels of IL-6 in the normal and SSc fibroblasts were increased by NE stimulation for one hour in a dose-dependent manner (Fig. 1A,B). Furthermore, the IL-6 mRNA and secreted protein levels in the SSc fibroblasts treated with NE were significantly higher than those observed in the normal fibroblasts treated with NE (Fig. 1A,B). Next, we analyzed the IL-6 mRNA and secreted protein levels in the normal and SSc fibroblasts treated with 10 μM of NE for the indicated time. The IL-6 mRNA levels in the normal and SSc fibroblasts increased after NE stimulation, peaking at one hour of stimulation, and then decreased to the basal values at three hours after stimulation (Fig. 1C). In addition, the IL-6 mRNA levels in the SSc fibroblasts treated with 10 μM of NE for one hour were significantly higher than those in the normal fibroblasts (Fig. 1C). The secreted protein levels of IL-6 from the normal and SSc fibroblasts were increased by NE treatment in a time-dependent manner (Fig. 1D), and the IL-6 secreted protein levels from the SSc fibroblasts treated with 10 μM of NE for six hours were significantly higher than those in the normal fibroblasts (Fig. 1D). These results suggest that NE enhances IL-6 production in normal and SSc fibroblasts in a dose- and time-dependent manner and that the NE-induced IL-6 production is significantly higher in SSc fibroblasts than in normal fibroblasts.
Figure 1. NE-induced IL-6 production via ARβ and NE-induced proliferation in SSc fibroblasts were significantly higher than those in normal fibroblasts.
(A,B) Effects of NE on IL-6 mRNA (A) and IL-6 protein (B) secreted into the media from normal and SSc fibroblasts. Normal and SSc fibroblasts were incubated during 1 hour with indicated concentration of NE. (C, D) Effects of NE on IL-6 mRNA (C) and IL-6 protein (D) secreted into the media from normal and SSc fibroblasts. Normal and SSc fibroblasts were incubated with 10 μM NE for indicated time. (E, F) IL-6 mRNA (E) and secreted protein (F) into the media in normal and SSc fibroblasts treated with oxymetazoline (Ox: ARα agonist) for 1 hour. (G,H) IL-6 mRNA (G) and secreted protein (H) into the media in normal and SSc fibroblasts treated with isoproterenol (Iso: ARβ agonist) for 1 hour. (I,J) IL-6 mRNA (I) and secreted protein (J) into the media in normal and SSc fibroblasts treated with NE (10 μM) and/or ARβ inhibitor, propranolol (Ppl) for 1 hour. n = 3-6 samples. mRNA levels in normal fibroblasts without treatments were assigned a value of 1. All values represent mean ± SEM. **P < 0.01, *P < 0.05.
NE-induced IL-6 production in both the normal and SSc fibroblasts was mediated primarily via ARβ
NE binds both ARα and ARβ to activate the downstream signaling. However, NE has different mechanisms of activation for ARα and ARβ, depending on the specific G protein subunits activated29,30. In order to examine whether the NE-induced IL-6 expression is conducted via ARα or ARβ, we assessed the IL-6 mRNA and secreted protein levels in normal and SSc fibroblasts treated with oxymetazoline (ARα agonist) or isoproterenol (ARβ agonist) for one hour. While oxymetazoline did not affect the IL-6 mRNA and secreted protein levels in the normal or SSc fibroblasts (Fig. 1E,F), isoproterenol elevated the IL-6 mRNA and secreted protein levels in both the normal and SSc fibroblasts (Fig. 1G,H). Next, we examined the effects of the ARβ inhibitor, propranolol, on NE-induced IL-6 production in normal and SSc fibroblasts. Consequently, propranolol significantly inhibited the NE-induced IL-6 mRNA and secreted protein levels in the SSc fibroblasts (Fig. 1IJ). These results suggest that the NE-induced IL-6 production in normal and SSc fibroblasts is mediated primarily via ARβ.
Expression of ARα and ARβ in the normal and SSc fibroblasts
Next, we explored the expression of AR in normal and SSc fibroblasts treated with or without NE. Consequently, NE did not affect the ARα1B, ARα1D or ARβ1 expression levels in the normal or SSc fibroblasts (Supplemental Figure S1A–C). There was a tendency for the ARβ2 expression to be higher in the normal and SSc fibroblasts treated with NE than in those treated without NE, however, there were no significant differences (Supplemental Figure S1D). In addition, there was a tendency for the ARα1 expression to be higher in SSc fibroblasts than in normal fibroblasts (Supplemental Figure S1A,B). However, there were no significant differences in the surface protein expression levels of ARα1 between the normal and SSc fibroblasts by immunofluorescence staining (Supplemental Figure S1E).
NE-induced phosphorylation of p38 via ARβ enhanced the IL-6 production in the SSc fibroblasts
It has been reported that stimulation of ARβ enhances the phosphorylation of p38 in cardiac fibroblasts31. In addition, p38 is known to be involved in the production of IL-6 in synovial and gingival fibroblasts32,33. These findings suggest that p38 signaling is associated with NE-induced IL-6 production in human dermal fibroblasts. In order to assess this hypothesis, we examined the phosphorylation of p38 in normal and SSc fibroblasts treated with NE, oxymetazoline or isoproterenol.
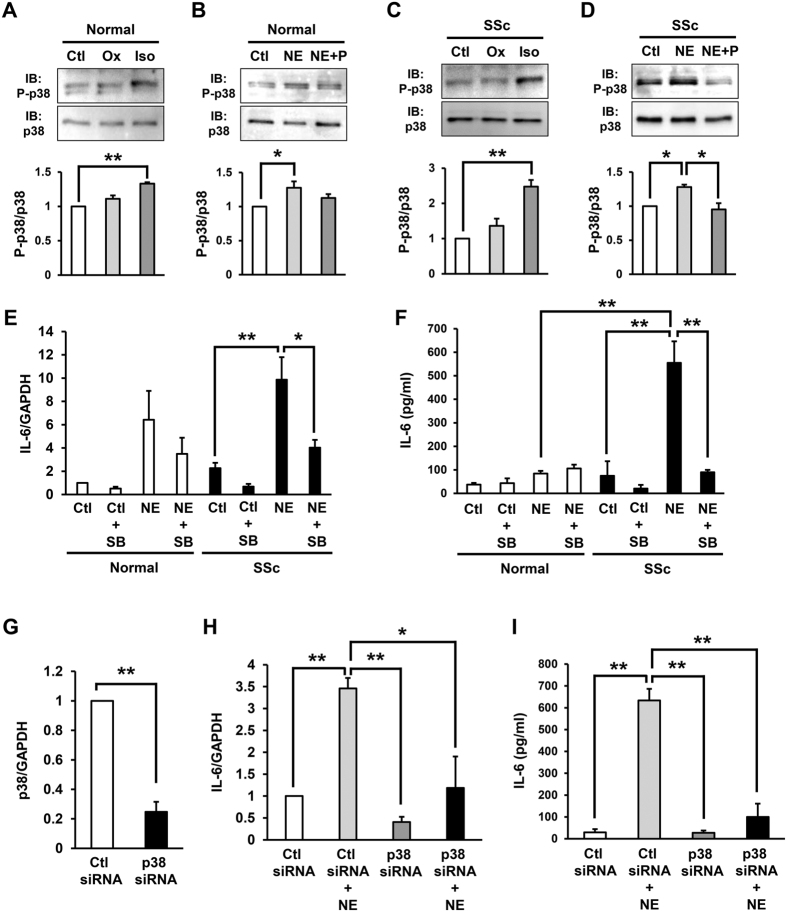
The phosphorylation of p38 in the normal and SSc fibroblasts was significantly enhanced by the ARβ agonist, isoproterenol, but not the ARα agonist, oxymetazoline (Fig. 2A,C). In addition, the phosphorylation of p38 in the normal and SSc fibroblasts was significantly enhanced by NE, and the NE-induced p38 phosphorylation was inhibited by the ARβ inhibitor, propranolol (Fig. 2B,D). These results suggest that NE-induced p38 phosphorylation is primarily mediated via ARβ. Furthermore, the NE-induced IL-6 mRNA and secreted protein levels in the SSc fibroblasts was significantly inhibited by the p38 inhibitor, SB203580 (Fig. 2E,F). Furthermore, to confirm the role of p38 in NE-induced IL-6 production, the effects of siRNA depletion of p38 in SSc fibroblasts were analysed. The expression of p38 mRNA in SSc fibroblasts treated with siRNA of p38 was reduced by approximately 80%, compared with that in the fibroblasts treated with control siRNA (Fig. 2G). siRNA depletion of p38 significantly inhibited NE-induced IL-6 mRNA and secreted protein levels in SSc fibroblasts (Fig. 2H,I). These results suggest that the NE-induced phosphorylation of p38 via ARβ enhances IL-6 production in SSc fibroblasts.
Figure 2. NE-induced phosphorylation of p38 via ARβ enhanced IL-6 production in SSc fibroblasts.
(A,C) Phosphorylation of p38 in normal (A) and SSc (C) fibroblasts treated with oxymetazoline (Ox) or isoproterenol (Iso) by immunoblotting. (B,D) Phosphorylation of p38 in normal (B) and SSc (D) fibroblasts treated with NE and/or ARβ inhibitor, propranolol (P) by immunoblotting. Quantification of relative phosphorylation levels of p38 was accomplished via densitometry using ImageJ. The level of p38 phosphorylation in normal or SSc fibroblasts without treatment was assigned a value of 1. n = 3 samples. Cropped blots were used, full-length blots are in Supplementary Figure S3. All gels have been run under the same experimental conditions. (E,F) IL-6 mRNA (E) and secreted protein (F) into the media in normal and SSc fibroblasts treated with NE (10 μM) and/or p38 inhibitor, SB203580 (SB) for 1 hour. n = 3 samples. mRNA levels in normal fibroblasts without treatments were assigned a value of 1. (G) p38 mRNA in SSc fibroblasts transfected with control or p38 siRNA. (H,I) IL-6 mRNA (H) and secreted protein (I) into the media in SSc fibroblasts transfected with control or p38 siRNA treated with or without NE. mRNA levels in control siRNA transfected SSc fibroblasts without treatments were assigned a value of 1. n = 3 samples. All values represent mean ± SEM. **P < 0.01, *P < 0.05.
NE-induced phosphorylation of ERK1/2 via ARα inhibited the IL-6 production in the SSc fibroblasts
Next, we examined the role of ERK1/2 in the NE-induced IL-6 production in fibroblasts. ERK1/2 is known to be involved in the downstream pathway of AR29,30. In this study, the phosphorylation of ERK1/2 in the normal and SSc fibroblasts was significantly enhanced by the ARα agonist, oxymetazoline, but not the ARβ agonist, isoproterenol (Fig. 3A,C). Moreover, the phosphorylation of ERK1/2 in the normal and SSc fibroblasts was enhanced by NE, while the NE-induced ERK1/2 phosphorylation was enhanced by the ARβ inhibitor, propranolol (Fig. 3B,D). These results suggest that NE-induced ERK1/2 phosphorylation is primarily mediated via ARα. Furthermore, the NE-induced IL-6 mRNA and secreted protein levels in the normal and SSc fibroblasts was enhanced by the MEK/ERK inhibitor, PD98059 (Fig. 3E,F), thus suggesting that the NE-induced phosphorylation of ERK1/2 via ARα inhibits IL-6 production in SSc fibroblasts.
Figure 3. NE-induced phosphorylation of ERK1/2 via ARα inhibited IL-6 production in SSc fibroblasts.
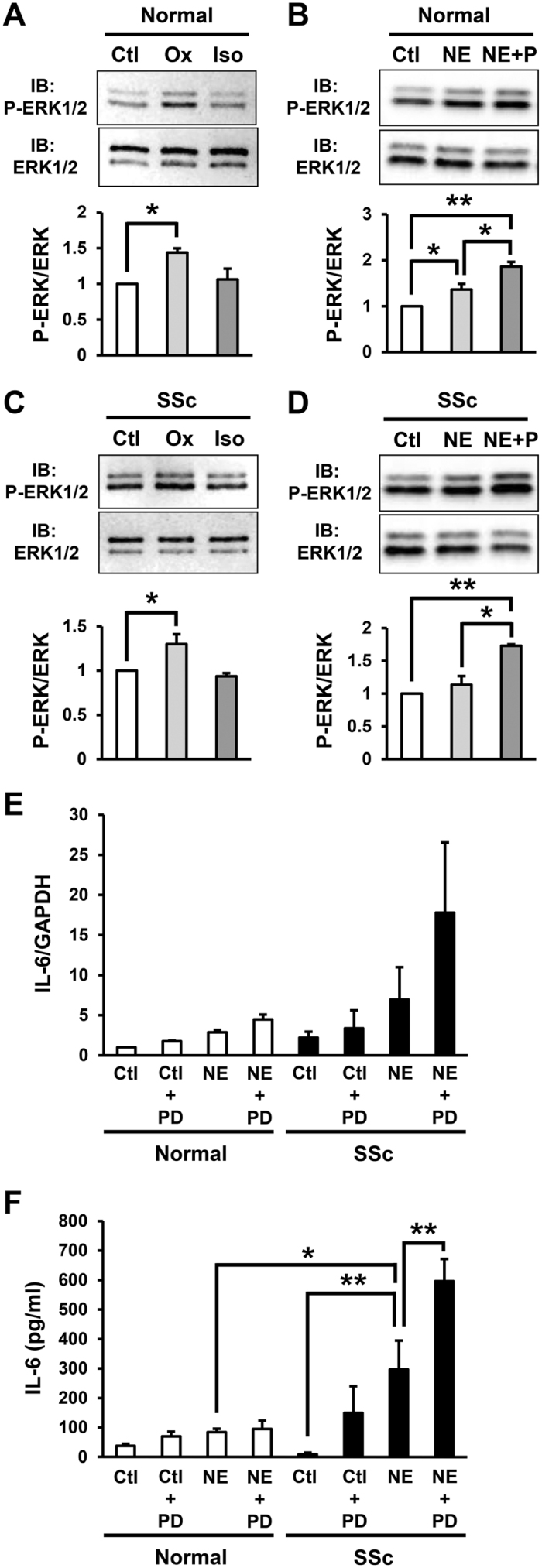
(A,C) Phosphorylation of ERK1/2 in normal (A) and SSc (C) fibroblasts treated with oxymetazoline (Ox) or isoproterenol (Iso) by immunoblotting. (B,D) Phosphorylation of ERK1/2 in normal (B) and SSc (D) fibroblasts treated with NE and/or ARβ inhibitor, propranolol (P) by immunoblotting. Quantification of relative phosphorylation levels of ERK1/2 was accomplished via densitometry using ImageJ. The level of ERK1/2 phosphorylation in normal or SSc fibroblasts without treatment was assigned a value of 1. n = 3 samples. Cropped blots were used, full-length blots are in Supplementary Figure S4. All gels have been run under the same experimental conditions. (E,F) IL-6 mRNA (E) and secreted protein (F) into the media in normal and SSc fibroblasts treated with NE and/or MEK/ERK inhibitor, PD98059 (PD) for 1 hour. n = 3 samples. mRNA levels in normal fibroblasts without treatments were assigned a value of 1. All values represent mean ± SEM. **P < 0.01, *P < 0.05.
Combined treatment with NE and ET-1 resulted in an additive increase in the production of IL-6 in the SSc fibroblasts
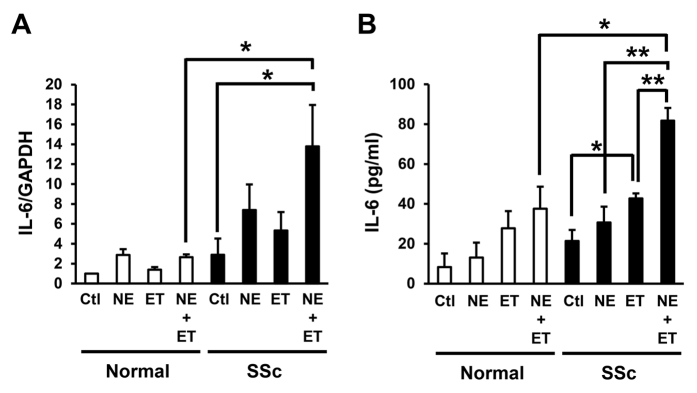
ET-1 has reported to be been involved in the pathogenesis of fibrosis in various organs, including the skin, lungs and heart34. ET-1 induces the production of collagen type I and III and fibronectin via ET receptors A and B on fibroblasts35. In addition, the plasma ET-1 levels are higher in patients with SSc than in normal subjects36, suggesting that ET-1 is associated with the pathogenesis of fibrosis in the setting of SSc. Therefore, we next examined the effects of ET-1 on the NE-induced IL-6 production in the normal and SSc fibroblasts. As a result, the IL-6 mRNA and protein levels in the SSc fibroblasts were enhanced by treatment with ET-1 for one hour (Fig. 4A,B). In addition, the IL-6 mRNA and protein levels were significantly enhanced by combined treatment with NE and ET-1 compared to that achieved with treatment with NE or ET-1 alone (Fig. 4A,B), suggesting that the NE-induced IL-6 production in SSc fibroblasts is additively enhanced by simultaneous treatment with ET-1.
Figure 4. Combined treatment with NE and ET-1 resulted in an additive increase in the production of IL-6 in SSc fibroblasts.
IL-6 mRNA (A) and secreted protein (B) into the media in normal and SSc fibroblasts treated with NE (10 μM) and/or ET-1 (1 μM) for 1 hour. n = 3 samples. mRNA levels in normal fibroblasts without treatments were assigned a value of 1. All values represent mean ± SEM. **P < 0.01, *P < 0.05.
NE-induced IL-6/IL-6 receptor trans-signaling increased the production of collagen type I in SSc fibroblasts
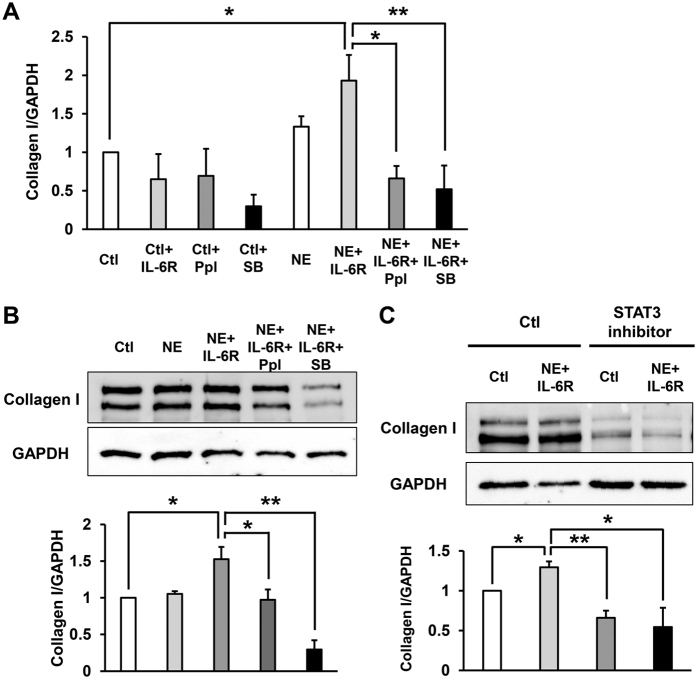
It has been reported that IL-6 complexes with a sIL-6R to associate with gp130 on the cell surface and initiate intracellular signaling, which is termed “trans-signaling”18,37,38. In addition, increased levels of serum IL-6 secreted from many cells interact with sIL-6R, enhancing activation of endothelial cells, expression of adhesion molecules and apoptosis, which seem to occur early in the development of SSc38. Therefore, we finally examined the role of NE-induced IL-6 and sIL-6R trans-signaling in fibrosis in SSc fibroblasts. Incubation of SSc fibroblasts with NE alone had no significant effect of both mRNA and protein levels of collagen production (Fig. 5A,B). However, incubation of SSc fibroblasts with NE and sIL-6R together significantly increased collagen type I production (Fig. 5A,B). In addition, the ARβ inhibitor, propranolol, and the p38 inhibitor, SB203580 significantly inhibited the NE and IL-6R-induced collagen type I production in the SSc fibroblasts (Fig. 5A,B). Furthermore, we examined the effect of STAT3 inhibitor, S31-201 on the NE-induced IL-6/IL-6R trans-signaling, and identified that STAT3 inhibitor significantly inhibited the NE and IL-6R-induced collagen type I production in the SSc fibroblasts (Fig. 5C). These results suggest that the NE-induced IL-6/IL-6R trans-signaling might enhance skin fibrosis in SSc, and ARβ inhibitor, propranolol, the p38 inhibitor, SB203580 and STAT3 inhibitor, S31-201 may suppress NE-induced skin fibrosis.
Figure 5. Combined treatment with NE and IL-6 receptor resulted in an increase in the production of collagen type I in SSc fibroblasts.
(A) Collagen type I mRNA in SSc fibroblasts treated with NE, sIL-6 receptor (IL-6R), propranolol (Ppl) or p38 inhibitor, SB203580 (SB) for 48 hours. n = 3 samples. mRNA levels in fibroblasts without treatments were assigned a value of 1. (B) Collagen type I in SSc fibroblasts treated with NE, sIL-6 receptor (IL-6R), propranolol or SB203580 for 48 hours by immunoblotting. (C) Collagen type I in SSc fibroblasts treated with NE, sIL-6 receptor (IL-6R) or STAT3 inhibitor (S31-201) for 48 hours by immunoblotting. Quantification of relative levels of collagen type I was accomplished via densitometry using ImageJ (normalized to GAPDH protein levels). The level of collagen type I in fibroblasts without treatment was assigned a value of 1. n = 3 samples. Cropped blots were used, full-length blots are in Supplementary Figure S5. All gels have been run under the same experimental conditions. All values represent mean ± SEM. **P < 0.01, *P < 0.05.
NE-induced proliferation was significantly higher in the SSc fibroblasts than in the normal fibroblasts
We then assessed the effects of NE on proliferation in the normal and SSc dermal fibroblasts. The degree of proliferation in the SSc fibroblasts was increased by treatment with NE (Supplemental Figure S2). In addition, the rate of proliferation was significantly higher in the SSc fibroblasts treated with NE than in the normal fibroblasts (Supplemental Figure S2). These results suggest that the NE-induced proliferation is significantly higher in SSc fibroblasts than in normal fibroblasts.
Discussion
There have been a number of reports suggesting the possible involvement of stress and/or the stress-adaptation system in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus (SLE)39,40. It has also been reported that the serum NE levels are significantly higher in SSc patients than in normal individuals1. In addition, the mental stress-induced serum NE levels are also higher in SSc patients than in normal individuals based on mental calculation stress tests, suggesting that SSc patients may have an impaired function of the neuro-endocrine-immune system due to stress1. Previous findings showed that serum NE levels in normal individuals were increased after cold exposure2, and serum NE levels increased after repeated cold-water immersions3, further indicate that emotional stress and cold exposure increase the systemic and/or local NE levels in SSc patients.
In this study, we demonstrated that NE stimulation increases the IL-6 expression in dermal fibroblasts in both normal and SSc patients and that NE enhances IL-6 production in SSc fibroblasts more so than in normal fibroblasts. Since the peripheral tissues of the extremities, including the fingers and toes, are likely to be exposed to cold stimulation, cold stimulation-induced NE may increase the IL-6 concentrations locally, resulting in peripheral skin sclerosis in SSc patients. This may also explain why the onset of skin fibrosis initially starts from the acral parts of the extremities. Furthermore, our results suggest that avoiding cold exposure or emotional stress may suppress both fibrosis and Raynaud’s phenomenon in SSc.
Moreover, we elucidated the mechanisms underlying the NE-induced IL-6 production in SSc fibroblasts and subsequently propose a model for the regulation of IL-6 production by NE in SSc fibroblasts (Fig. 6). In this study, the production of IL-6 in the SSc fibroblasts was enhanced by both NE and the ARβ agonist, isoproterenol, but not the ARα agonist, oxymetazoline, suggesting that the NE-induced IL-6 production is primarily mediated via ARβ. NE binds to and activates the downstream signaling of ARβ, thus leading to the phosphorylation of p38. This NE-induced activation of p38 signaling may consequently enhance IL-6 production in SSc fibroblasts. In contrast, the NE-induced phosphorylation of ERK1/2 via ARα subsequently results in the inhibition of IL-6 production in SSc fibroblasts. It is thought that IL-6 is able to bind to the soluble IL-6 receptor secreted from B cells, T cells and monocytes and thus form a complex with gp130 on fibroblasts that enhances collagen production18,20,21,22,23. In addition, it has been recognized that STAT3 is important in the IL-6/IL-6R trans-signaling in fibroblasts41. We demonstrated that NE-induced IL-6/IL-6 receptor trans-signaling via STAT3 activation increased collagen type I production in SSc fibroblasts, suggesting that NE induces IL-6 secretion from dermal fibroblasts and has both paracrine and autocrine effects on various cells, including dermal fibroblasts, T cells and B cells, that stimulate skin fibrosis in the setting of SSc. The NE-induced proliferation of fibroblasts may also lead to skin fibrosis in SSc patients. Further elucidating the regulatory mechanisms mediated by NE:AR signaling may provide new insight into the pathogenesis of skin sclerosis associated with SSc.
Figure 6. Model for the regulation of IL-6 production by NE in SSc fibroblasts.
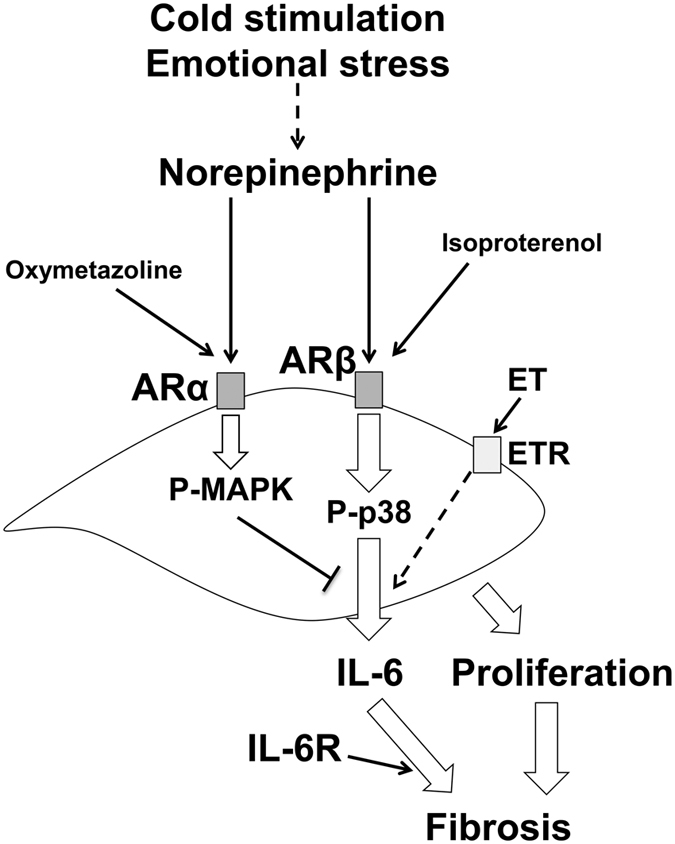
Emotional stress and/or cold exposure might increase systemic and/or local NE levels. Production of IL-6 in SSc fibroblasts was enhanced by both NE and ARβ agonist, isoproterenol, but not ARα agonist, oxymetazoline, suggesting that NE-induced IL-6 production is primarily mediated via ARβ. NE binds to and activates the downstream signaling of ARβ, thus leading to the phosphorylation of p38. This NE-induced activation of p38 signaling may consequently enhance IL-6 production in SSc fibroblasts. In contrast, the NE-induced phosphorylation of ERK1/2 via ARα subsequently results in the inhibition of IL-6 production in SSc fibroblasts. NE-induced IL-6 production and proliferation of fibroblasts might lead to skin fibrosis in SSc. ET: endothelin. ETR: endothelin receptor.
It has been known that ARβ stimulation increases cyclic AMP (cAMP), leading to the activation of protein kinase A (PKA)42. Wang et al. reported that prostaglandin E2 enhanced IL-6 production via cAMP/PKA pathway in human chondrocytes, suggesting that NE-induced IL-6 might be mediated via cAMP/PKA pathway43. However, we have not examined the involvement of cAMP/PKA pathway in the present study, therefore, further examinations are needed.
In addition, the NE-enhanced IL-6 production and proliferation are greater in SSc fibroblasts than in normal fibroblasts. We examined AR expression in normal and SSc fibroblasts and found that there were no significant differences between normal and SSc fibroblasts with or without NE treatment. These results suggest that the AR expression is not associated with the mechanisms underlying the higher NE-induced IL-6 production observed in SSc fibroblasts than in normal fibroblasts. The mechanisms underlying the hyperreactivity of IL-6 production in SSc fibroblasts treated with NE are unknown, and further studies are thus required to clarify these processes.
In this study, we found that the NE-induced IL-6 production in SSc fibroblasts is additively enhanced by treatment with ET-1, suggesting that NE:ARβ signaling and ET-1:ET receptor signaling additively enhance IL-6 production in these cells. Similar to our results, it has been previously reported that ET-1, acting via the ET receptor A and PKC activation, is an essential co-factor for the ARβ-induced proliferation of human cardiac fibroblasts44. The current findings provide new insight into the relationship between NE:AR signaling and ET-1:ET receptor signaling in SSc fibroblasts, although further investigations are also required to elucidate the precise mechanisms underlying the additive effects of ET-1 on the NE-induced IL-6 production in SSc fibroblasts.
In conclusion, we herein demonstrated that NE enhances IL-6 production in SSc fibroblasts via ARβ, subsequently enhances collagen production in SSc fibroblasts. p38 activation is involved in this mechanism, and both cold exposure and emotional stress may initiate this process. We for the first time elucidated the possible link between peripheral circulation disturbance and tissue fibrosis via through NE stimulation, which will provide us with novel therapeutic strategy for SSc. The avoidance of cold exposure or emotional stress may attribute to the suppression of fibrosis as well as Raynaud’s phenomenon in SSc. In addition, we speculate that cold exposure-induced NE may explain the reason why the vasculopathy and skin fibrosis initially start from acral parts of extremities in SSc patients. In the current study, the ARβ blocker, propranolol, the p38 inhibitor, SB203580, and STAT3 inhibitor, S31-201 inhibited the NE-induced IL-6 production and fibrosis in SSc fibroblasts in vitro, suggesting that ARβ blocker, p38 and/or STAT3 inhibitor therapy can be an alternative treatment for skin sclerosis in patients with SSc45.
Methods
Reagents
We used (±)-norepinephrine (+)-bitartrate salt (Sigma-Aldrich, St Louis, MO), isoproterenol hydrochloride (Millipore, Billerica, MA), propranolol hydrochloride (Wako, Osaka, Japan), oxymetazoline hydrochloride (Wako), p38 inhibitor, SB203580 (Wako), MEK/ERK inhibitor, PD98059 (Millipore), STAT3 inhibitor, S31-201 (Sigma-Aldrich), recombinant human TGFβ1 (rTGFβ) (R&D systems, Minneapolis, MN), ET-1 (Sigma-Aldrich) and soluble IL-6 receptor (sIL-6R) (Peprotech, Rocky Hill, NJ).
Patients
We obtained human dermal fibroblasts by skin biopsies of affected dorsal forearm areas from 6 dcSSc patients and age, race and gender matched 6 healthy volunteers. All SSc patients were <2 years of skin thickening and fulfilled the criteria for SSc proposed by American College of Rheumatology46. The study was approved by the research ethics committee/human genome, gene analysis research ethics committee of Gunma University (#185). All patients and volunteers provided written informed consent before participation. This study was conducted according to the Declaration of Helsinki principles.
Cell cultures
Human dermal fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 units/ml penicillin, 100μg/ml streptomycin and 10% fetal calf serum (FCS) and used before passage 10. Human dermal fibroblasts were incubated in DMEM with or without NE, oxymetazoline or isoproterenol at indicated concentration for indicated time. To examine the effect of the ARβ inhibitor, propranolol, the MEK/ERK inhibitor, PD98059, p38 inhibitor, SB203580 or STAT3 inhibitor, S31-201 on NE-induced IL-6 production, cells were pretreated with 1 μM propranolol, 50 μM PD98059, 10 μM SB203580 or 100 μM S31-201 or DMSO (vehicle control) for 30 minutes, and then stimulated with NE (10 μM) and/or sIL-6 receptor (IL-6R: 100 ng/ml). The concentration of NE (0-10 μM), propranolol (1 μM), PD98059 (50 μM), SB203580 (10 μM) and S31-201 (100 μM) was referred from previously reported manuscripts31,47,48,49,50. To inhibit the expression of p38, fibroblasts were transfected with 100 nM p38 siRNA (Cell Signaling Technology, Danvers, MA). After 48 hours, mRNA levels were assessed by quantitative RT-PCR. Fibroblasts were treated with NE (10 μM) for 1 hour at 48 hours after transfection of siRNA.
Real-time polymerase chain reaction (PCR) analysis
Total RNA was isolated by RNeasy Mini Kits (Qiagen, Valencia, CA) and was subjected to reverse transcription with the use of a Superscript III First-Strand Synthesis System for reverse transcription (RT)-PCR (Invitrogen) according to previously described protocols49,51. Quantitative RT-PCR was performed using the Taqman system (Applied Biosystems, Foster City, CA) using 7300 Real Time PCR systems (Applied Biosystems) according to the manufacturer’s instructions. Taqman probes and primers for IL-6, ARα1B, ARα1D, ARβ1, ARβ2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. As an internal control, levels of GAPDH were quantified in parallel with target genes. Normalization and fold changes were calculated using the comparative Ct method.
Measurement of IL-6 concentration
The specific enzyme-linked immunosorbent assay (ELISA) kit was used for measuring IL-6 (R&D systems) in supernatants of the culture according to the manufacture’s protocol.
Western blot assay
Western blotting and analyses were performed according to previously described protocols49. Human dermal fibroblasts were incubated in normal medium with or without oxymetazoline (1 μM) or isoproterenol (1 μM) for 1 hour. Cells were pretreated with 1 μM propranolol, 10 μM SB203580, 100 μM S31-201 or DMSO (vehicle control) for 30 minutes, and then stimulated with NE (10 μM) and/or sIL-6 receptor (IL-6R: 100 ng/ml). After washing with ice-cold PBS, cells were disrupted in lysis buffer (20 mM Tris-HCl pH 7.6, 140 mM NaCl, 1% Nonidet P-40) containing 1 mM phenylmethylsulfonyluoride, aprotinin (10 mg/ml) and 1 mM sodium vanadate. Lysates were centrifuged at 10,000 × g for 15 min at 4 °C and the resulting supernatants were subjected to SDS-PAGE, followed by immunoblot analysis using anti-phospho-p38 MAPK (Thr180/Tyr182) Ab, anti-p38 MAPK Ab, anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Ab, anti-44/42 MAPK (Erk1/2) Ab (Cell Signaling, Danvers, MA), anti-collagen type I Ab (SouthernBiotech, Birmingham, AL) and anti-GAPDH Ab (Santa Cruz Biotechnology, Dallas, Texas). Anti-rabbit HRP-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used with ECL (Thermo Scientific, Rockford, IL) to image immunoblots. Quantification of protein band intensity was performed using ImageJ software version 1.46r (NIH) as previously reported49.
Proliferation assays
Human dermal fibroblasts (5 × 105 cells per well) were plated in a 6 cm2 dish and starved in serum free medium overnight. Cells were treated with or without NE (10 μM). After 48 hours incubation at 37 °C, cells were counted by Coulter particle Counter (Beckman coulter, Brea, CA).
Immunofluorescence staining
Immunofluorescence staining were performed according to previously described protocols49. Human dermal fibroblasts were treated with or without NE (10 μM) for 1 hour, and then fixed in 4% paraformaldehyde (PFA) in PBS for 30 minutes. After being blocked with 3% drymilk–PBS supplemented with 5% normal goat serum for 1 hour, cells were stained with rabbit anti-α1AR antibody (Invitrogen) followed by Alexa 488–conjugated secondary Ab (Invitrogen). Cells were mounted in ProLong Gold antifade reagent (Invitrogen).
Statistical analysis
P values were calculated by analysis of one-way ANOVA followed by Bonferroni’s post test. Error bars represent standard errors of the mean, and numbers of experiments (n) are as indicated.
Additional Information
How to cite this article: Uehara, A. et al. Mechanistic insight into the norepinephrine-induced fibrosis in systemic sclerosis. Sci. Rep. 6, 34012; doi: 10.1038/srep34012 (2016).
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 15K19675.
Footnotes
Author Contributions A.U. and S.-i.M. involved in all the process of planning and implementing the experiment, interpretation of data, and writing the manuscripts. K.Y., A.U., B.P., S.T., S.O., Y.Y. and Y.T. involved in the process of implementing the experiment. O.I. involved in the process of planning the experiment, interpretation of data, and writing the manuscripts. All authors reviewed the manuscript.
References
- Matsuura E. et al. Functional disturbance of the stress-adaptation system in patients with scleroderma. Mod Rheumatol 21, 397–405 (2011). [DOI] [PubMed] [Google Scholar]
- Leppäluoto J., Korhonen I. & Hassi J. Habituation of thermal sensations, skin temperatures, and norepinephrine in men exposed to cold air. J Appl Physiol 90, 1211–1218 (2001). [DOI] [PubMed] [Google Scholar]
- Young, , Muza S. R., Sawka M. N., Gonzalez R. R. & Pandolf K. B. Human thermoregulatory responses to cold air are altered by repeated cold water immersion. J Appl Physiol 60, 1542–1548 (1985). [DOI] [PubMed] [Google Scholar]
- Steinkraus V. et al. Autoradiographic mapping of beta-adrenoceptors in human skin. Arch Dermatol Res 288, 549–553 (1996). [DOI] [PubMed] [Google Scholar]
- McSwigan J. D., Hanson D. R., Lubiniecki A., Heston L. L. & Sheppard J. R. Down syndrome fibroblasts are hyperresponsive to beta-adrenergic stimulation. Proc Natl Acad Sci USA 78, 7670–7673 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillbro J. M., Marles L. K., Hibberts N. A. & Schallreuter K. U. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol 123, 346–353 (2004). [DOI] [PubMed] [Google Scholar]
- Romana-Souza B., Otranto M., Almeida T. F., Porto L. C. & Monte-Alto-Costa A. Stress-induced epinephrine levels compromise murine dermal fibroblast activity through β-adrenoceptors. Exp Dermatol 20, 413–419 (2011). [DOI] [PubMed] [Google Scholar]
- Pullar C. E. et al. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 132, 2076–2084 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar C. E. & Isseroff R. R. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci 119, 592–602 (2006). [DOI] [PubMed] [Google Scholar]
- Wigley F. M. Clinical practice. Raynaud’s Phenomenon. N Engl J Med 347, 1001–1008 (2002). [DOI] [PubMed] [Google Scholar]
- Herrick A. L. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol 8, 469–479 (2012). [DOI] [PubMed] [Google Scholar]
- Wise, et al. Efficacy and tolerability of a selective alpha(2C)-adrenergic receptor blocker in recovery from cold-induced vasospasm in scleroderma patients: a single-center, double-blind, placebo-controlled, randomized crossover study. Arthritis Rheum 50, 3994–4001 (2004). [DOI] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T. & Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today 11, 443–449 (1990). [DOI] [PubMed] [Google Scholar]
- Hirano T. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324, 73–76 (1986). [DOI] [PubMed] [Google Scholar]
- Bettelli E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- Ishihara K. & Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 13, 357–368 (2002). [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Littlewood A. J., Maddison P. J. & Hall N. D. Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol 13, 17–22 (1995). [PubMed] [Google Scholar]
- Khan K. et al. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann Rheum Dis 71, 1235–1242 (2012). [DOI] [PubMed] [Google Scholar]
- Sato S., Hasegawa M. & Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Der Sci 27, 140–146 (2001). [DOI] [PubMed] [Google Scholar]
- Hügle T. et al. Tumor necrosis factor-costimulated T lymphocytes from patients with systemic sclerosis trigger collagen production in fibroblasts. Arthritis Rheum 65, 481–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali C. A., Bost K. L., Boulware D. W. & Levy L. S. Mechanisms of pathogenesis in scleroderma. I.Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol 19, 1207–1211 (1992). [PubMed] [Google Scholar]
- Kawaguchi Y., Hara M. & Wright T. M. Endogenous IL-1alpha from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest 103, 1253–1260 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T. C., Anderson M. E. & Moots R. J. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol 2011, 721608 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford) 49, 2408–2412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016). [DOI] [PubMed] [Google Scholar]
- Takemura H. et al. Enhanced interleukin 6 production by cultured fibroblasts from patients with systemic sclerosis in response to platelet derived growth factor. J Rheumatol 25, 1534–1539 (1998). [PubMed] [Google Scholar]
- Kadono T., Kikuchi K., Ihn H., Takehara K. & Tamaki K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J Rheumatol 25, 296–301 (1998). [PubMed] [Google Scholar]
- Fukasawa C. et al. Increased CD40 expression in skin fibroblasts from patients with systemic sclerosis (SSc): role of CD40-CD154 in the phenotype of SSc fibroblasts. Eur J Immunol 33, 2792–2800 (2003). [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. M., Rasmussen S. G. & Kobilka B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S., Rajagopal K. & Lefkowitz R. J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9, 373–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. et al. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol 40, 384–393 (2006). [DOI] [PubMed] [Google Scholar]
- Jin J. et al. Different signaling mechanisms regulating IL-6 expression by LPS between gingival fibroblasts and mononuclear cells: seeking the common target. Clin Immunol 143, 188–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. H., Chiu Y. C., Tan T. W., Yang R. S. & Fu W. M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol 179, 5483–5492 (2007). [DOI] [PubMed] [Google Scholar]
- Teder P. & Noble P. W. A cytokine reborn? Endothelin-1 in pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 23, 7–10 (2000). [DOI] [PubMed] [Google Scholar]
- Horstmeyer A., Licht C., Scherr G., Eckes B. & Krieg T. Signalling and regulation of collagen I synthesis by ET-1 and TGF-beta1. FEBS J 272, 6297–6309 (2005). [DOI] [PubMed] [Google Scholar]
- Yamane K. et al. Significance of plasma endothelin-1 levels in patients with systemic sclerosis. J Rheumatol 19, 1566–1571 (1992). [PubMed] [Google Scholar]
- Le T. T. et al. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J Immunol 193, 3755–3768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muangchan C. & Pope J. E. Interleukin 6 in systemic sclerosis and potential implications for targeted therapy. J Rheumatol 39, 1120–1124 (2012). [DOI] [PubMed] [Google Scholar]
- Chikanza I. C., Petrou P., Kingsley G., Chrousos G. & Panayi G. S. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum 35, 1281–1288 (1992). [DOI] [PubMed] [Google Scholar]
- Hirano D., Nagashima M., Ogawa R. & Yoshino S. Serum levels of interleukin 6 and stress related substances indicate mental stress condition in patients with rheumatoid arthritis. J Rheumatol 28, 490–495 (2001). [PubMed] [Google Scholar]
- O’Reilly S., Ciechomska M., Cant R. & van Laar J. M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem 289, 9952–9960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G. The beta-adrenergic receptors. Herz 27, 683–690 (2002). [DOI] [PubMed] [Google Scholar]
- Wang P., Zhu F. & Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol 298, C1445–1456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N. A., O’regan D. J., Ball S. G. & Porter K. E. Endothelin-1 is an essential co-factor for beta2-adrenergic receptor-induced proliferation of human cardiac fibroblasts. FEBS Lett 576, 156–160 (2004). [DOI] [PubMed] [Google Scholar]
- O’Reilly S., Ciechomska M., Cant R., Hügle, & van Laar J. M. Interleukin-6, its role in fibrosing conditions. Cytokine Growth Factor Rev 23, 99–107 (2012). [DOI] [PubMed] [Google Scholar]
- Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23, 581–590 (1980). [DOI] [PubMed] [Google Scholar]
- Lai K. B., Sanderson J. E. & Yu C. M. The regulatory effect of norepinephrine on connective tissue growth factor (CTGF) and vascular endothelial growth factor (VEGF) expression in cultured cardiac fibroblasts. Int J Cardiol 163, 183–189 (2013). [DOI] [PubMed] [Google Scholar]
- Franke J. & Abraham G. Concomitant inhibition of primary equine bronchial fibroblast proliferation and differentiation by selective β2-adrenoceptor agonists and dexamethasone. Eur J Pharmacol 741, 205–213 (2014). [DOI] [PubMed] [Google Scholar]
- Motegi S. et al. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arterioscler Thromb Vasc Biol 31, 2024–2034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. et al. NSC 74859-mediated inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinoma. Liver Int 32, 70–77 (2012). [DOI] [PubMed] [Google Scholar]
- Motegi S., Garfield S., Feng X., Sárdy M. & Udey M. C. Potentiation of platelet-derived growth factor receptor-β signaling mediated by integrin-associated MFG-E8. Arterioscler Thromb Vasc Biol 31, 2653–2664 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.