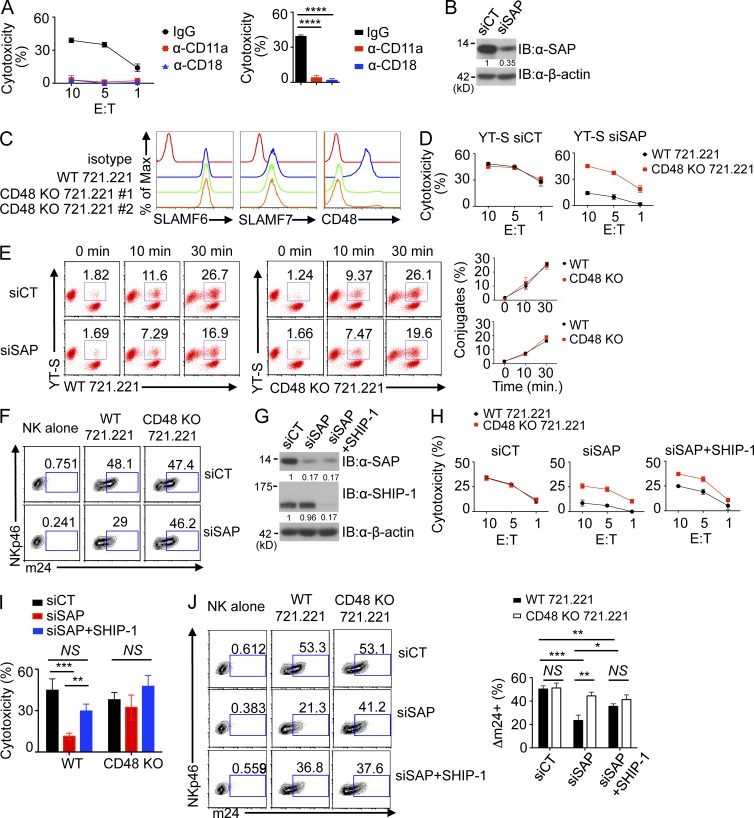
Figure 10.
2B4-mediated inhibition results in decreased activation of LFA-1. (A, left) The ability of the human NK cell line YT-S to mediate cytotoxicity of the B cell line 721.221 was assessed at the indicated E/T ratio in the presence of blocking antibodies against CD11a (LFA-1) or CD18 or control IgG. Standard deviations of duplicates are shown by error bars. (Right) Statistical analyses of results at the 10:1 E/T ratio for three independent experiments are shown. ****, P < 0.0001. (B) Expression of SAP in YT-S cells transfected with scrambled siRNA (control [siCT]) or siRNAs against SAP (siSAP) was analyzed by immunoblot (IB) analysis. Results are representative of four experiments. (C) Expression of SLAMF6, SLAMF7, and CD48 was analyzed on parental (WT) or CD48 KO 721.221 cells. Two different targeting sequences were used to generate two different CD48 KO derivatives of 721.221 through CRISPR-Cas–mediated genome editing. Isotype control is shown in red. Results are representative of three experiments. (D) Natural cytotoxicity of YT-S cells transfected with control siRNAs (left) or siRNAs against SAP (right) was analyzed toward wild-type or CD48 KO 721.221 cells at the indicated E/T ratio. For CD48 KO 721.221 cells, a pool of CD48 KO 721.221 no. 1 and no. 2 cells was used. Mean values of duplicates with standard deviations are depicted. Results are representative of four experiments. (E) YT-S cells transfected with control or SAP-specific siRNAs were labeled with Brilliant violet 421–conjugated anti–human NKp46 and then incubated for the indicated times at 37°C with parental (WT) or CD48-deficient 721.221 cells labeled with PE-conjugated anti–human CD19. Conjugate formation was assessed by flow cytometry. A representative experiment is depicted on the left, whereas a statistical analysis of duplicates with mean values and standard deviations is shown on the right. Data are representative of three experiments. (F) YT-S cells transfected with control siRNAs or SAP-specific siRNAs were incubated with medium alone, wild-type 721.221, or CD48 KO 721.221 for 5 min at 37°C. After stimulation, cells were stained with anti-CD11a conformational antibody (m24) for 30 min at room temperature, followed by anti-NKp46 staining for 15 min at room temperature. YT-S cells were gated on NKp46+ cells and analyzed for m24 staining. Results are representative of three experiments. (G) Expression of SAP and SHIP-1 in YT-S cells transfected with control siRNA, siRNAs against SAP, or SHIP-1 was analyzed by immunoblot analysis. β-actin was used as the control. Results are representative of three experiments. (H) Natural cytotoxicity of YT-S cells transfected with the siRNAs detailed in G was determined at the indicated E/T ratio toward wild-type or CD48 KO 721.221 cells. (I) Statistical analyses of the results at the 10:1 E/T ratio from multiple independent experiments are shown. (J, left) YT-S cells transfected with the siRNAs detailed in G were incubated with medium alone, WT 721.221, or CD48 KO 721.221 and stained with the anti-CD11a conformational antibody (m24), as outlined for F. (Right) Statistical analyses of the results from multiple independent experiments are shown. (H–J) *, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired Student’s t test. Mean values with standard deviations are depicted. Results are representative of four experiments.