Fig. 5.
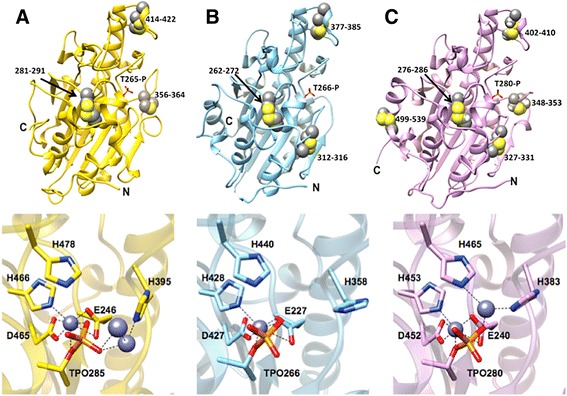
Comparison of the crystal structures of the catalytic domains of MCR-1 (a), EptC (C. jejuni) (PDB code 4TN0) (b), and LptA (N. meningitidis) (PDB code 4KAY) PEA transferases and their conserved active-site residues (c). Top panels – All of the enzymes adopt a similar fold and the active-site threonine is in phosphorylated form. The active site phosphothreonine is labeled for each enzyme. Disulfide bonds are shown as space-fill spheres and the numbers of the participating residues are labeled. Bottom panels – Representative active-site residues are shown in stick model together with interacting zinc ions represented as slate blue spheres. The dashed lines represent interacting distances < 3.3 Å. The MCR-1 active site has three zinc ions bound compared to one zinc for EptC and two zincs for LptA. The MCR-1 zinc ion that is coordinated by phosphothreonine-285, Glu246, Asp465, and His466 is conserved in EptC and LptA
