Abstract
The overexpression of urokinase-type plasminogen activator receptor (uPAR) is associated with inflammation and virtually all human cancers. Despite the fact that docosahexaenoic acid (DHA) has been reported to possess anti-inflammatory and anti-tumor properties, the negative regulation of uPAR by DHA is still undefined. Here, we investigated the effect of DHA on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced uPAR expression and the underlying molecular mechanisms in ECV304 human endothelial cells. DHA concentration-dependently inhibited TPA-induced uPAR. Specific inhibitors and mutagenesis studies showed that PKCδ, JNK1/2, Erk1/2, NF-κB, and AP-1 were critical for TPA-induced uPAR expression. Application of DHA suppressed TPA-induced translocation of PKCδ, activation of the JNK1/2 and Erk1/2 signaling pathways, and subsequent AP-1 and NF-κB transactivation. In conclusion, these observations suggest a novel role for DHA in reducing uPAR expression and cell invasion by inhibition of PKCδ, JNK1/2, and Erk1/2, and the reduction of AP-1 and NF-κB activation in ECV304 human endothelial cells.
Introduction
Tumor metastasis is the most common cause of poor prognosis and deaths in cancer patients. Urokinase-type plasminogen activator (uPA) and urokinase-type plasminogen activator receptor (uPAR) system is thought to play a role in tumor angiogenesis [1] and tumor metastasis [2]. uPAR is over-expressed in tumors by multiple tumor-associated cell types including the tumor cells themselves, stromal cells and endothelial cells [3]. Coordination of extracellular matrix proteolysis and cell signaling by uPAR underlies its important function in tumor metastasis and make it an attractive therapeutic target in cancer [4]. uPAR appears to also elicit a plethora of cellular responses include cellular adhesion, differentiation, proliferation and migration [5, 6]. Moreover, uPAR expression increase with grade of tumor and maybe enriched in metastatic lesions [7]. The expression of a catalytically inactive enzyme or an antisense uPAR cDNA, which results in the decreasing of uPAR decreases cell invasiveness [8]. Therefore, agents with the ability to block uPAR expression may hold potential as treatments for human cancers. The level of uPAR expression is stimulated by a diverse set of agents, including vascular endothelial growth factor [9], epidermal growth factor [10], hepatocyte growth factor [11], and fibroblast growth factor [12] in a number of different cell types. uPAR is also stimulated by hypoxia in breast cancer cells [13].
TPA, a potent tumor promoter, stimulates renal tumor cell proliferation through activation of protein kinase C (PKC) [14]. TPA-induced uPAR is mediated by the activation of MAPK signaling pathways and transcription factors such as NF-κB and AP-1 in human ovarian and gastric cancer cells [15, 16]. Because of the critical roles of PKC, MAPKs, NF-κB, and AP-1 in TPA-induced uPAR expression and cancer cell metastasis, substances that inhibit these factors may confer anti-tumor activity.
Polyunsaturated fatty acids (PUFAs) can be divided into two major groups: ω-3 and ω-6 PUFAs [17]. Docosahexaenoic acid (DHA), a major ω-3 PUFAs that is enriched in fatty fish and fish oil supplements, is well known for its anti-inflammatory and anticancer properties [18, 19]. Regarding its anticancer effect, DHA was reported to inhibit MMP-9 expression in human breast cancer MCF-7 cells [20]. In addition, DHA has been shown to reduce monocyte chemoattractant-1 (MCP-1) through PPARγ and NF-κB in human epithelial cells [21].
In the present study, we aimed to investigate DHA’s effect on TPA-induced uPAR expression in ECV304 human endothelial cells, and to reveal its underlying molecular mechanisms.
Materials and Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), OPTI-modified Eagle’s medium, fetal bovine serum (FBS), phosphate buffered saline, and penicillin–streptomycin solution were obtained from HyClone (Logan, UT, USA). TrypLE™ Express was obtained from Gibco (Grand Island, NY, USA). The bicinchoninic acid protein assay kit was from Pierce (Rockford, IL, USA). TPA, DMSO, LY294002 hydrochloride, curcumin, rottlerin, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). BAY11-7082, PD98059, SP600125, and SB203580 were purchased from Calbiochem (San Diego, CA, USA). Antibodies against uPAR, PKCδ, phos-PKCδ (Tyr 311), phos-Akt (Ser 473), Akt, phos-JNK1/2, JNK1/2, phos-Erk1/2, Erk1/2, phos-p38, p38, phos-c-jun, phos-c-fos, phos-p65 (Ser 536), phos-IκBα (Ser 32), and IкBα were purchased from Cell Signaling Technology (Danvers, MA, USA), and antibodies against c-jun, c-fos and Clathrin HC were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The ECV304 human endothelial cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 0.6% penicillin–streptomycin at 37°C in a 5% CO2 humidified incubator. In these experiments, stimulants such as TPA, were added to serum-free media for the indicated time intervals. When the inhibitors were used, they were added 1 h before the TPA treatment.
Cell viability assay
Cell viability after treatment was determined by the MTT assay. Cells were incubated with 1mg/ml MTT for 3 h, and subsequently solubilized in DMSO. The presence of DHA or the other chemicals did not interfere with the measurement at 570 nm wavelength measured using a microplate spectrophotometer (Epoch, Biotek, USA).
Isolation of cell fractions
The cells were harvested and then washed twice with ice-cold PBS. Homogenization buffer A (200 μL; 20 mM Tris–HCl, pH8.0, 10 mM EGTA, 2 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25 μg/mL aprotinin, and 10 μg/mL leupeptin) was added to each dish, and the cells were scraped into a 1.5 mL tube. Cells were centrifuged at 5000× g for 15 min at 4°C. The cell pellet was collected as the nuclear fraction. The supernatant was centrifuged at 15,000×g at 4°C for 60 min to yield the pellet (membrane fraction) and the supernatant (cytosolic fraction).
Reverse transcription PCR, and real-time PCR
Total cellular RNA was extracted from cells using RNAiso Reagent (TaKaRa Bio, Otsu, Japan). The complementary DNA was subjected to PCR amplification with the primer sets for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and uPAR, using a PCR master mix solution (iNtRON, Seongnam, Gyeonggi-do, Korea). The specific primer sequences were GAPDH sense, 5′-TTG TTG CCA TCA ATG ACCCC-3′; GAPDH antisense, 5′-TGA CAA AGT GGT CGT TGA GG-3′(836 bp); uPAR sense, 5′-CAC GAT CGT GCG CTT GTG GG-3′; and uPAR antisense, 5′-TGT TCT TCA GGG CTG CGG CA-3′ (285 bp). The PCR conditions included denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 45 s. The products were electrophoresed in a 1.5% agarose gel containing ethidium bromide. PCR product formation was monitored continuously during the reaction using Sequence Detection System software, version 1.7 (Applied Biosystems, Foster City, CA, USA). Accumulated PCR products were detected directly by monitoring the increase of the reporter dye (SYBR®). The mRNA expression levels of uPAR in the treated cells were compared to the expression levels in control cells at each time point using the comparative cycle threshold (Ct)-method [22]. The quantity of each transcript was calculated as described in the instrument manual and normalized to the amount of GAPDH, a housekeeping gene.
Western blot analysis
After each experiment, cells were washed twice with cold PBS and were harvested in 100 μL of protein extraction solution (iNtRON, Seongnam, Gyeonggi-do, Korea). Cell homogenates were centrifuged at 10,000×g for 20 min at 4°C. Equal amounts of total cellular protein (50 μg) were electrophoresed in sodium dodecyl sulfate (SDS)-polyacrylamide gels, and the protein was then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Nonspecific binding sites on the membranes were blocked with 5% nonfat dry milk in 15 mM Tris/150 mM NaCl buffer (pH 7.4) at room temperature for 2 h. Membranes were incubated with target antibody. The membranes were then probed with secondary antibody labeled with horseradish peroxidase. The bands were visualized using an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA) and were scanned by a luminescence image analyzer (Vilber Lourmat, France).
Transient transfection with siRNAs and dominant negative mutants
Stealth RNAi duplexes corresponding to human siRNAs of PKC, PKCδ, and Akt were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The plasmids encoding dominant negative mutants of MEK-1 (pMCL-K97M), JNK (pMCL-TAM67), and p38 MAPK (pMCL-mP38) were kindly provided by Dr. N.G. Ahn (University of Colorado, Boulder, CO, USA), Dr. M.J. Birrer (NCI, Rockville, MD, USA), and Dr. J. Han (Scripps Research Institute, CA, USA), respectively. The phosphorothioated double-stranded oligodeoxynucleotide (ODNs) with sequences targeting the AP-1 binding site (5'-CAC TCA GAA GTC ACT TC-3' and 3'-GAA GTG ACT TCT GAG CTG-5') were prepared (Genotech, St. Louis, MO, USA) and annealed (AP-1 decoy ODNs). The dominant negative mutants of I-κBα and I-κBβ and NIK were kindly provided by Dr. D.W. Ballard (Vanderbilt University, Nashville, TN, USA) and Dr. W.C. Greene (University of California, CA, USA), respectively. All mutants were prepared by using Qiagen (Valencia, CA, USA) plasmid DNA preparation kits. Transient transfections of siRNAs (100 nM) and dominant negative mutants (1 μg) were carried out using Lipofectamine 2000 from Invitrogen (Carlsbad, CA, USA).
Measurement of uPAR, AP- 1 and NF-κB luciferase activity
The plasmid pGL3/uPAR-promoter was generous gift from Dr. Y. Wang (Australian National University, Canberra, Australia). The NF-κB and AP-1 luciferase reporter plasmid was purchased from Clontech (Palo Alto, CA, USA). ECV304 were seeded and grown until they reached 70% confluence. Then, cells were co-transfected with siRNAs of PKC, PKCδ, Akt, scrambled sequence, uPAR luciferase. PRL-TK was transfected as an internal control. Cells were collected with cell culture lysis reagent (Promega, Madison, WI, USA) and the luciferase activity was determined using a luminometer (Centro XS lb960 microplate luminometer,Berthold Technologies, USA) according to the manufacturer’s protocol.
Matrigel invasion assay
The cell invasion assay was carried out using 10-well chemotaxis chambers (Neuro Probe, Gaithersburg, Maryland, USA) with an 8-μM pore membrane (Neuro Probe) in DMEM with 10% FBS as the chemoattractant in the lower chamber. The non-invading cells on the upper surface of each membrane were removed from the chamber by using cotton swabs, and the invading cells on the lower surface of each membrane were stained using the Quick-Diff stain kit (Becton-Dickinson, Franklin Lakes, NJ, USA). After two washes with water, the chambers were allowed to air dry. The number of invading cells was counted using a phase-contrast microscope.
Statistics analysis
Data are shown as the mean ± standard deviation (SD) and represent the mean of at least three separate experiments performed in triplicate. Differences between data sets were determined by t-tests. Differences described as significant in the text correspond to P values of<0.05.
Results
DHA inhibits TPA-induced uPAR in ECV304 cells
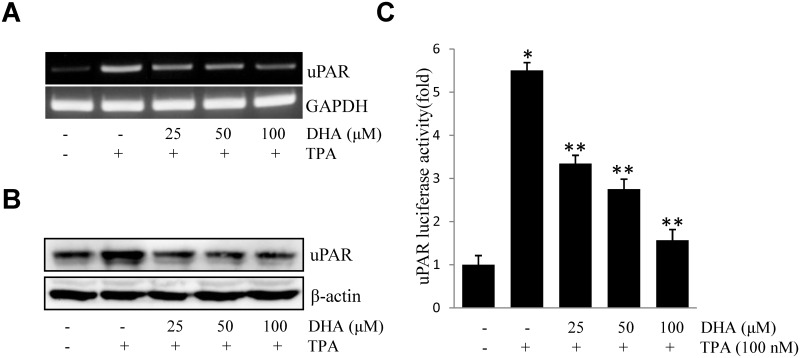
To investigate the suppressive effect of DHA on the up-regulation of uPAR, ECV304 cells pretreated with DHA were incubated with TPA. TPA-stimulated uPAR mRNA expression (Fig 1A), protein expression (Fig 1B), and promoter activity (Fig 1C) were inhibited by DHA in a concentration-dependent manner as illustrated. These results suggested that DHA inhibited TPA-induced uPAR expression in ECV304 cells.
Fig 1. DHA inhibits TPA-induced uPAR in ECV304 cells.
Cells were pretreated with DHA (25, 50, 100 μM) for 1 h, followed by incubation with 100 nM TPA for 4 or 16 h. uPAR mRNA level (A), protein level (B), and promoter activity (C) were measured by RT-PCR, western blot, and luciferase activity assay, respectively. *P<0.05 versus control; **P<0.05 versus TPA only. The data represent the mean ± SD from triplicate measurements.
DHA inhibits TPA-induced uPAR by suppressing PKCδ activation
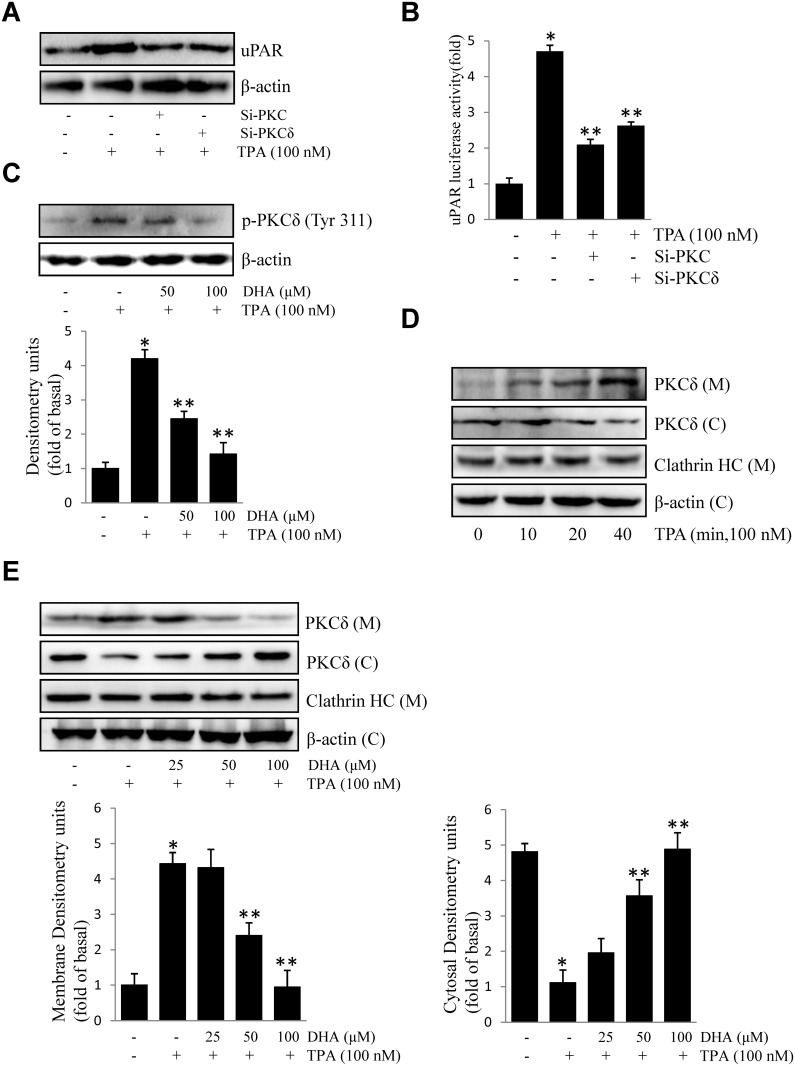
Activation of PKCs has been shown to correlate with tumor metastasis [23]. However, contributions of PKC isoforms to TPA-induced uPAR in ECV304 cells are still unclear. As shown in Fig 2A and 2B, transfection of si-PKC and si-PKCδ inhibited TPA-induced uPAR protein expression and promoter activity. Next, we found that DHA inhibits TPA-induced phosphorylation of PKCδ (Fig 2C). Activation of PKC by TPA involves in the translocation of PKC isoforms to the plasma membrane. Translocation of the PKCδ protein from the cytosol to the membrane was detected in TPA-treated cells, but was blocked by the addition of DHA (Fig 2D and 2E). This illustrated that PKCδ activation is involved in TPA-induced uPAR, which was inhibited by the addition of DHA.
Fig 2. DHA inhibits TPA-induced uPAR via suppression of PKCδ.
(A) si-PKC or si-PKCδ were transfected into cells. After incubation with 100 nM TPA for 16 h, uPAR levels were evaluated by western blotting. (B) si-PKC or si-PKCδ were co-transfected with PGL3-uPAR into cells. After incubation with 100 nM TPA for 4 h, luciferase activity was measured using a luminometer. (C) Cells were treated with DHA (50, 100 μM) followed by TPA for 20 min, and the phosphorylation of PKCδ was analyzed by western blotting. (D) Cells were treated with TPA for 10–40 min, the subcellular components of cells were extracted, and the levels of PKCδ in the cytosolic and membrane fractions were analyzed by western blotting. (E) Cells were treated with DHA (25, 50, 100 μM) followed by TPA for 20 min, and PKCδ in the cytosol and membrane was evaluated as described above. *P<0.05 versus control; **P<0.05 versus TPA only. The data represent the mean ± SD from triplicate measurements.
DHA suppresses Erk1/2 and JNK1/2 activation downstream of PKCδ
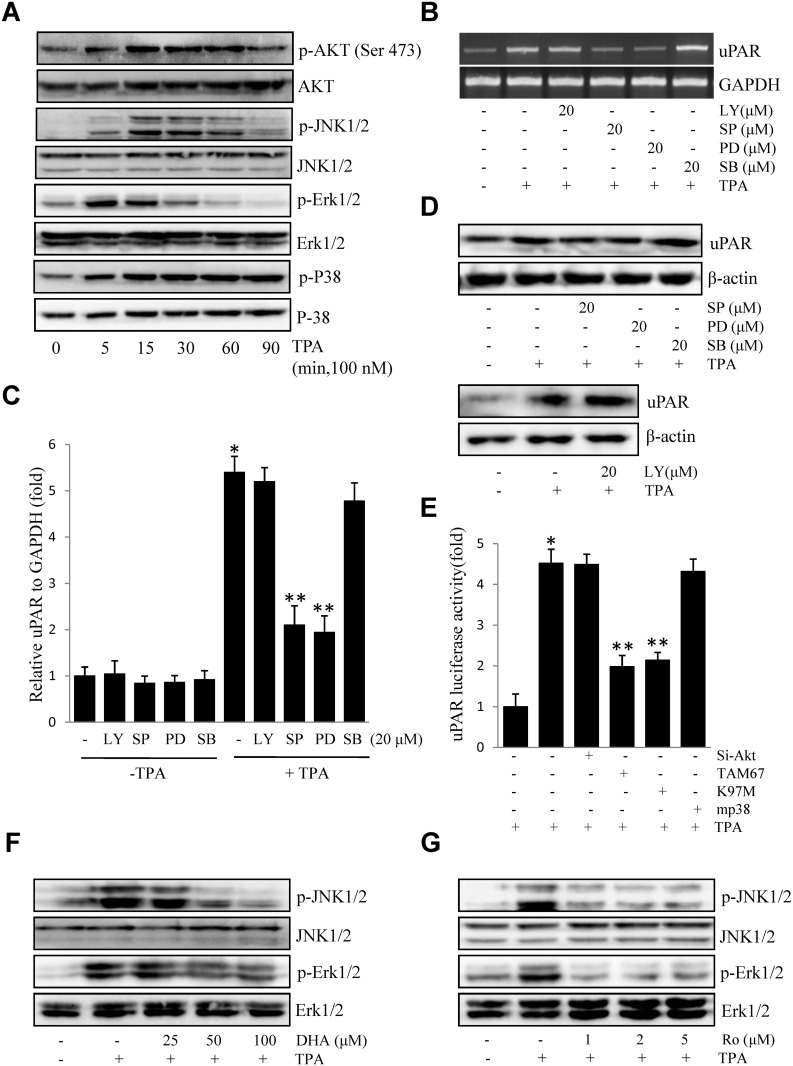
To determine the signaling molecules involved in TPA-induced uPAR expression, we investigated the levels of phosphorylated Akt and changes in MAPKs (Erk1/2, JNK1/2, and P38 MAPK) in ECV304 cells exposed to TPA for various periods. As shown in Fig 3A, induction of Akt, JNK1/2, and Erk1/2, and p38 phosphorylation elicited by TPA were detected. Pharmacological inhibitors of Akt and MAPK were used to determine the molecular mechanisms by which TPA induces uPAR expression. As shown in Fig 3B and 3C, treatment of SP (a JNK inhibitor) and PD (an Erk inhibitor) decreased TPA-induced uPAR mRNA expression, whereas treatment with LY or SB did not. As expected, treatment of SP and PD also decreased TPA-induced uPAR protein expression (Fig 3D). Consistent with these results, dominant negative mutants of JNK (TAM67) and MEK-1 (K97M) inhibited TPA-induced uPAR promoter activity. However, si-Akt or the dominant negative mutants of p38 MAPK (mp38) showed no effect (Fig 3E). These findings demonstrated that uPAR induction by TPA was mediated through JNK1/2 and Erk1/2 activation, and that these were blocked by pretreatment with DHA (Fig 3F). Furthermore, phosphorylation of JNK1/2 and Erk1/2 was blocked by treatment with rottlerin (a PKCδ inhibitor) (Fig 3G), suggesting that DHA suppression of JNK1/2 and Erk1/2 occurs downstream of PKCδ.
Fig 3. DHA inhibits TPA-induced uPAR via suppression of JNK1/2 and Erk1/2.
(A) Cells were incubated with 100 nM TPA for 0–90 min, and cell lysates were blotted using specific antibodies. (B) Cells pretreated with LY (20 μM), SP (20 μM), PD (20 μM), or SB (20 μM) for 1h were incubated with 100 nM TPA for 4 h. After incubation, uPAR mRNA levels were determined by RT-PCR. (C) Cells were pretreated with LY (20 μM), SP (20 μM), PD (20 μM), or SB (20 μM) for 1 h in the presence or absence of 100 nM TPA for 4 h. After incubation, uPAR mRNA levels were determined by real-time PCR. (D) Cells pretreated with SP (20 μM), PD (20 μM), SB (20 μM) or LY (20 μM) for 1 h were incubated with 100 nM TPA for 16 h. After incubation, uPAR protein levels were determined by western blotting. (E) si-Akt, dominant negative mutants of JNK (TAM67), MEK-1(K97M), or mutant p38 MAPK (mp38) were co-transfected with PGL3-uPAR into cells. After incubation with 100 nM TPA for 4 h, luciferase activity was measured using a luminometer. (F) Cells were treated with DHA (25, 50 and 100 μM), followed by TPA treatment for 15 min, and cell lysates were blotted using specific antibodies. (G) Cells were treated with Ro (1, 2, and 5 μM), followed by TPA treatment for 15 min. Expressions of phosphorylated JNK1/2 and phosphorylated Erk1/2 were evaluated by western blotting. *P<0.05 versus control; **P<0.05 versus TPA only. The data represent the mean ± SD from triplicate measurements.
DHA inhibits TPA-induced uPAR by suppressing DNA-binding activities of AP-1
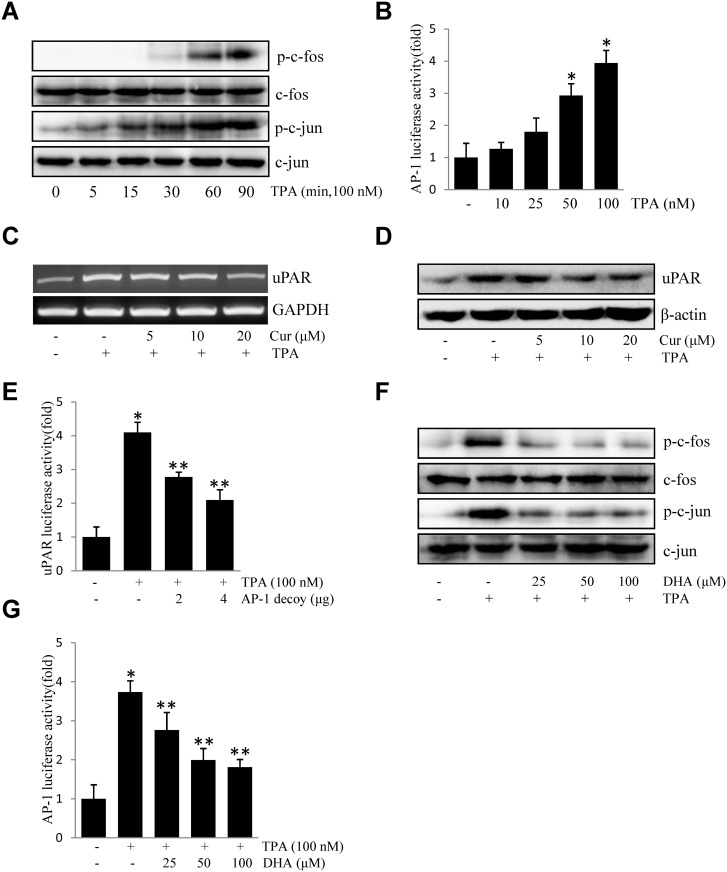
Accumulating evidence showed that AP-1 plays a pivotal role in tumorigenesis [24]. To study the role of transcription factors AP-1 in TPA-induced uPAR expression, the effect of TPA on the activation of AP-1 was investigated in ECV304 cells. As shown in Fig 4A, TPA treatment induced the phosphorylation of c-fos and c-jun, both of which are members of the AP-1 family. Consistently, TPA treatment resulted in an increase in AP-1-dependent transcriptional activity in cells transiently transfected with the AP-1 luciferase reporter construct (Fig 4B). Moreover, treatment of cells with 5–20 μM curcumin, an AP-1 inhibitor, suppressed uPAR mRNA (Fig 4C) and protein expression (Fig 4D). Similarly, when ECV304 cells were transiently transfected with an AP-1 decoy, ODN, TPA-induced uPAR promoter activity was decreased by the decoy oligonucleotide in a dose-dependent manner (Fig 4E). Pretreatment with DHA resulted in significant inhibition of TPA-induced activation of c-fos and c-jun (Fig 4F). AP-1 promoter activity was also identified (Fig 4G). These results suggested that DHA inhibits TPA-induced uPAR through the suppression of AP-1 activation.
Fig 4. DHA inhibits TPA-induced uPAR by suppressing the DNA-binding activities of AP-1 in ECV304 cells.
(A) Cells were treated with TPA for 0–90 min, and the cellular extracts were blotted using specific antibodies. (B) Cells were transiently transfected with the pAP-1 luciferase reporter construct. The transfected cells were incubated with TPA for 4 h and the luciferase activities were determined using a luminometer. (C) Cells were treated with 0–20 μM curcumin (Cur) for 1 h prior to exposure to 100 nM TPA for 4 h. After incubation, the uPAR mRNA levels in the cell lysates were determined by RT-PCR. (D) Cells were treated with 0–20 μM curcumin (Cur) for 1 h prior to exposure to 100 nM TPA for 16 h. After incubation, the uPAR protein levels were determined western blotting. (E) The AP-1 decoy oligonucleotide was co-transfected with pGL3-uPAR into cells. After incubation with 100 nM TPA for 4 h, the luciferase activities were determined using a luminometer. (F) Cells were treated with DHA (25, 50, 100 μM) prior exposure to 100 nM TPA, and the expressions of phos-c-fos, phos-c-jun were analyzed by western blotting. (G) Cells were transiently transfected with the pAP-1 luciferase reporter construct, after being pretreated with DHA (25, 50, 100 μM), and then were incubated with 100 nM TPA for 4 h. After incubation, the cells were lysed and luciferase activity was determined. *P<0.05 versus control; **P<0.05 versus only TPA. The data represent the mean ± SD from triplicate measurements.
DHA inhibits TPA-induced uPAR by suppressing DNA-binding activities of NF-кB p65
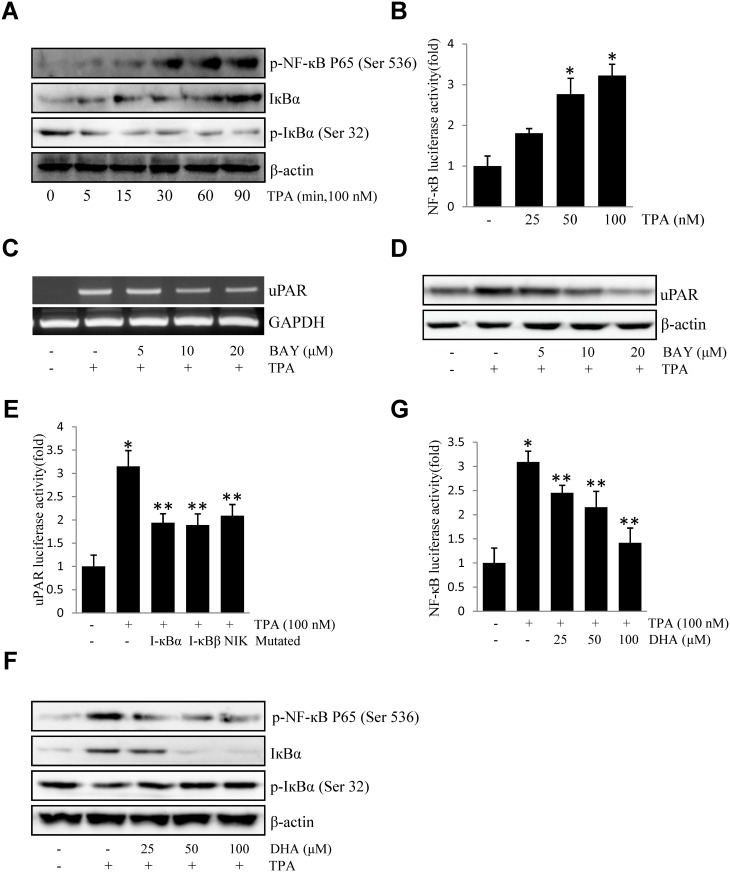
NF-κB is a pleiotropic, multifunctional transcription factor, involved in cancer proliferation, migration and apoptosis [25, 26]. Activation of NF-κB is usually associated with the induction of IκB phosphorylation; as expected, TPA enhanced the activation of serine 536-phosphorylated NF-κB p65 and serine32-phosphorylated I-κBα and caused the degradation of IκBα in ECV304 cells (Fig 5A). Furthermore, TPA increased transcriptional activity of NF-κB in a concentration-dependent manner (Fig 5B). BAY11-7082 (a NF-κB inhibitor) pretreatment decreased TPA-induced expression of uPAR mRNA (Fig 5C) and protein expression (Fig 5D). Additionally, the expression of dominant negative mutant forms of I-κBα, I-κBβ, or NIK resulted in a decrease in TPA-induced uPAR promoter activity (Fig 5E). Moreover, DHA blocked the activation of serine 536-phosphorylated NF-κB p65 and serine32-phosphorylated I-κBα (Fig 5F) and NF-B promoter activity (Fig 5G). Together, the above data implied that DHA inhibits TPA-induced uPAR through the suppression of NF-κB p65 activation.
Fig 5. DHA inhibits TPA-induced uPAR by suppressing the DNA-binding activities of NF-кB p65 in ECV304 cells.
(A) Cells were treated with TPA for 0–90 min, and the cellular extracts were blotted using specific antibodies. (B) Cells were transiently transfected with the pNF-кB luciferase reporter construct. The transfected cells were incubated with TPA for 4 h and the luciferase activities were determined using a luminometer. (C) Cells were treated with 0–10 μM BAY11-7082 for 1 h prior to exposure to 100 nM TPA for 4 h. After incubation, the uPAR mRNA levels in the cell lysates were determined by RT-PCR. (D) Cells were treated with 0–10 μM BAY11-7082 for 1 h prior to exposure to 100 nM TPA for 16 h. After incubation, the uPAR protein levels were determined by western blotting. (E) The dominant negative mutant of I-κBα, I-κBβ, and NIK were co-transfected with pGL3-uPAR into cells. After incubation with 100 nM TPA for 4 h, the luciferase activities were determined using a luminometer. (F) Cells were treated with DHA (25, 50, 100 μM) prior exposure to 100 nM TPA, and the expressions of phos-p65 (Ser 536), phos-IкB-α (Ser 32), and IкB-α were analyzed by western blotting. (G) Cells were transiently transfected with the pNF-кB luciferase reporter construct, after being pretreated with DHA (25, 50, 100 μM), and then were incubated with 100 nM TPA for 4 h. After incubation, the cells were lysed and luciferase activity was determined. *P<0.05 versus control; **P<0.05 versus only TPA. The data represent the mean ± SD from triplicate measurements.
DHA inhibits TPA-induced cell invasiveness
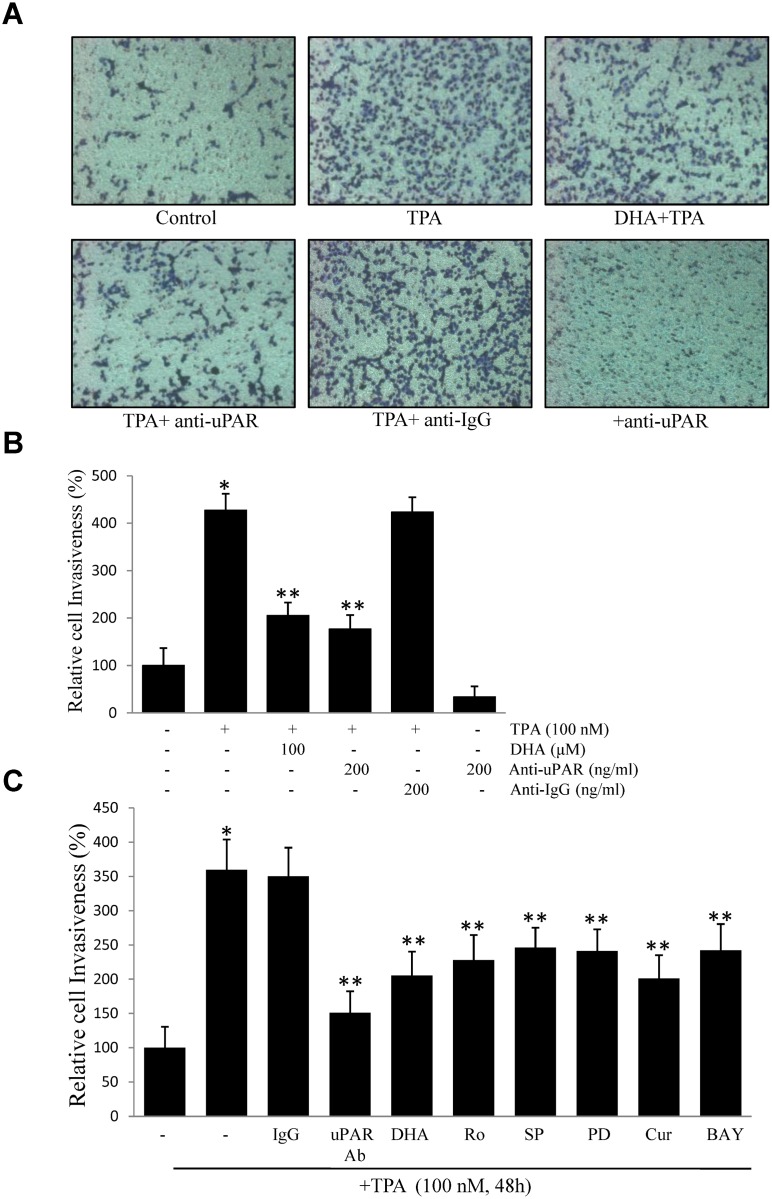
To examine the effect of DHA on TPA-induced cell invasion, we examined cell invasion through a modified Boyden invasion chamber. Incubated of ECV304 cells in TPA resulted in an increased number of invasive cells that passed through the artificial matrigel. However, in the presence of DHA or uPAR antibody, the number of invasive cells decreased, suggesting that DHA may suppress TPA-induced cell invasiveness by inhibiting uPAR expression in ECV304 cells (Fig 6A and 6B). Next, we investigated the effect of a signaling inhibitor on TPA-induced cell invasion. As shown in Fig 6C, uPAR antibody, DHA, Ro, SP, PD, Cur, and BAY inhibited cell invasion induced by TPA, indicating that DHA probably inhibits TPA-induced uPAR via suppression of PKCδ, JNK1/2, and Erk1/2, and reduction of AP-1 and NF-κB activation in ECV304 cells.
Fig 6. DHA inhibits ECV304 cell invasion by suppressing uPAR.
(A) (B) Cells (105) were incubated with 100 nM TPA in the presence or absence of 100 μM DHA or 200 ng/mL uPAR antibody or 200 ng/ml IgG antibody in a BIOCOAT™Matrigel apparatus for 48 h. (C) Cells (105) were incubated with 100 nM TPA in the presence of non-specific IgG (200 ng/mL), anti-uPAR antibody (200 ng/mL), 2 μM Ro, 20 μM PD, 20 μM SP, 20 μM Cur, and 20 μM BAY. After incubation, the cells invaded the undersurface of the chambers and were counted using a phase contrast light microscope after staining with a Diff-Quick Stain Kit. *P<0.05 versus control; **P<0.05 versus TPA only. The data represent the mean ± SD from triplicate measurements.
Discussion
Cancer has attracted considerable attention in recent decades, because it is a leading cause of death globally [27]. Much effort has been directed at defining the role of DHA as a cancer chemopreventive agent in humans. This interest has been stimulated by the following observations. i) The ω-3 PUFAs are important constituents of cell membranes that play multiple roles in regulating membrane fluidity, eicosanoid synthesis, cell signaling, and gene expression [28]. Ye et al reported that DHA reduces oxidative stress induced calcium influx by altering lipid composition in membrane caveolar rafts [29]. ii) DHA modulates multiple molecular pathways. DHA was reported to activate large-conductance Ca2+- dependent K+ channels [30]. iii) DHA are natural ligands of several nuclear receptors and transcription factors that regulate gene expression in some tissues [31]. iv) Accumulating evidence indicates that DHA inhibits various genes, including VEGF and COX-2, that are related to inflammation and tumor metastasis [32–34]. v) DHA enhances chemotherapy. In one study, DHA was shown to increase butyrate-mediated apoptosis through promoter methylation [35]. Additionally, DHA is essential for normal brain growth and cognitive function [36]. In this study, we explored the effects of DHA on uPAR expression and cell invasion in ECV304 human endothelial cells. Our results provide novel evidence that DHA effectively inhibits TPA-induced uPAR and cell invasion.
TPA, a protein kinase activator, has been used as a tumor promoter in chemical-induced carcinogenesis in vitro and in vivo. Several studies indicate that up-regulation and activation of PKCs are highly correlated with tumor metastasis [23, 37]. In the present study, using a PKC si-RNA (si-PKC) and PKCδ si-RNA (si-PKCδ), attenuated TPA-induced uPAR, and the ability of DHA to suppress TPA-induced translocation of PKCδ from the cytosol to the plasma membrane may have reduced the metastatic potential. It is noteworthy that PKC degrades with chronic TPA treatment [14], which deserves further rigorous research. MAPKs comprise a highly conserved cascade of serine/threonine kinases connecting cell surface receptors to regulatory targets in response to various stimuli [38]. Pharmacological studies have shown that incubation of ECV304 cells with JNK1/2 inhibitor or Erk1/2 inhibitor attenuated TPA-induced uPAR and that expression of JNK1/2 and Erk1/2 could be diminished by DHA treatment. EGFR is known to play a role in TPA-induced glioblastoma cell proliferation [23, 39]. EGFR was also reported to serve as downstream element in the signaling triggered by uPAR [40]. The Src tyrosine kinase has well established roles in the expression of uPAR and progression of human cancers [41, 42]. In this respect, many additional signaling modulators should be investigated to explore DHA suppression of TPA-induced uPAR and cell invasiveness in ECV304 cells.
Our results agree with earlier reports regarding the role of NF-кB and AP-1 in uPAR expression by macrophage-stimulating protein in gastric cancer AGS cells [43]. AP-1 is composed of members of the c-fos and c-jun families, which have been shown to regulate the expression of a number of genes involved in tumorigenesis. Here, activation of c-fos and c-jun was observed in TPA-treated cells, and they may be key molecules involved in uPAR expression in ECV304 cells. Moreover, DHA’s significant suppression of phosphrylation of c-fos and c-jun accompanied by a reduction in AP-1 transcription factor activity therefore inhibited uPAR expression. The sequestration of NF-κB by IκB in the cytoplasm and IκB phosphorylation leading to the proteasomal degradation of IκBα results in activation and translocation of NF-κB into the nucleus, and it is essential for the expression of several genes [44]. Treatment with DHA attenuated TPA-induced NF-кB DNA binding complex formation, and these results were consistent with a recent study [21]. A prior study suggested that the EGFR signaling activates NF-кB via mTORC2 [45]. The upstream signaling for AP-1 was Erk1/2 and JNK1/2 in cadmium-induced ECV304 cells [46]. It is likely that cross-talk between reactive oxygen species (ROS) and NF-кB might exit and modulate cellular signaling events [47]. Furthermore, the transcription factor SP1 binds to the uPAR promoter [48].
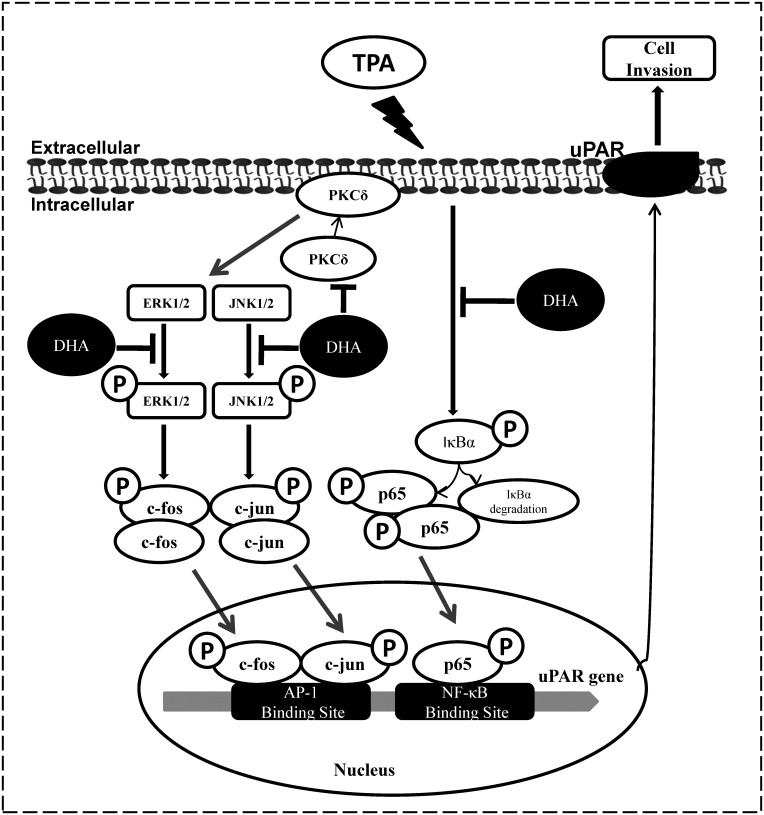
In summary, as shown in Fig 7, our results suggest that DHA inhibits TPA-induced uPAR expression and that it is, at least in part, involved in the inhibition of the PKCδ, JNK1/2 and Erk1/2, signaling pathways and in the reduction of AP-1 and NF-κB transcriptional activation. DHA may represent a novel target molecule or therapeutic approach to repress cancer progression.
Fig 7. Scheme summarizing the mechanisms of DHA inhibition of TPA-induced uPAR in human endothelial ECV304 cells.
DHA inhibits uPAR expression and cell invasion by inhibition of PKCδ, JNK1/2, and Erk1/2, and the reduction of AP-1 and NF-κB activation.
Acknowledgments
This study was supported by a research grant (0720570) from the National Cancer Center, by a Basic Science Research Program grant through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2015R1D1A1A09057240), and by a Medical Research Center (2012-000-9442) grant from the Korean Science and Engineering Foundation.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a Research grant (0720570) from the National Cancer Center (www.ncc.re.kr), Basic Science Research Program grant through the National Research Foundation of Korea (NRF) funded by the ministry of Education, Science, and Technology (2015R1D1A1A09057240, www.moe.go.kr), and a Medical Research Center (2012-000-9442) grant from the Korean Science and Engineering Education (www.nrf.re.kr). All above funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. THROMBOSIS AND HAEMOSTASIS-STUTTGART-. 2007;97(3):336. [PubMed] [Google Scholar]
- 2.Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer and Metastasis Reviews. 2003;22(2–3):205–22. [DOI] [PubMed] [Google Scholar]
- 3.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clinical cancer research. 2008;14(18):5649–55. 10.1158/1078-0432.CCR-07-4863 [DOI] [PubMed] [Google Scholar]
- 4.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nature reviews Molecular cell biology. 2010;11(1):23–36. 10.1038/nrm2821 [DOI] [PubMed] [Google Scholar]
- 5.Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N. The urokinase-system-role of cell proliferation and apoptosis. 2008. [DOI] [PubMed] [Google Scholar]
- 6.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nature reviews Molecular cell biology. 2002;3(12):932–43. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Hayashi Y, Wang Y, Nakamura T, Morita Y, Kawasaki K, et al. Urokinase type plasminogen activator receptor expression in colorectal neoplasms. Gut. 1998;43(6):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Liang X, Wu S, Murrell GA, Doe WF. Inhibition of colon cancer metastasis by a 3′-end antisense urokinase receptor mRNA in a nude mouse model. International journal of cancer. 2001;92(2):257–62. [DOI] [PubMed] [Google Scholar]
- 9.Baker E, Bergin F, Leaper D. Plasminogen activator system, vascular endothelial growth factor, and colorectal cancer progression. Journal of Clinical Pathology. 2000;53(6):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek MK, Kim MH, Jang HJ, Park JS, Chung IJ, Shin BA, et al. EGF stimulates uPAR expression and cell invasiveness through ERK, AP-1, and NF-κB signaling in human gastric carcinoma cells. Oncology reports. 2008;20(6):1569–75. [PubMed] [Google Scholar]
- 11.Nishimura K, Matsumiya K, Miura H, Tsujimura A, Nonomura N, Matsumoto K, et al. Effects of hepatocyte growth factor on urokinase-type plasminogen activator (uPA) and uPA receptor in DU145 prostate cancer cells. International journal of andrology. 2003;26(3):175–9. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Abe T, Wakabayashi Y, Hikawa T, Matsuo K-i, Yamada Y, et al. Up-regulation of urokinase-type plasminogen activator and its receptor correlates with enhanced invasion activity of human glioma cells mediated by transforming growth factor-α or basic fibroblast growth factor. Journal of neuro-oncology. 2000;46(2):115–23. [DOI] [PubMed] [Google Scholar]
- 13.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial–mesenchymal transition in hypoxic breast cancer cells. The Journal of cell biology. 2007;178(3):425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb TM, Davis MA. The tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) provokes a prolonged morphologic response and ERK activation in Tsc2-null renal tumor cells. Toxicological Sciences. 2004;81(1):233–42. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Suzuki M, Kanayama N, Nishida T, Takigawa M, Terao T. Suppression of urokinase receptor expression by bikunin is associated with inhibition of upstream targets of extracellular signal-regulated kinase-dependent cascade. European Journal of Biochemistry. 2002;269(16):3945–57. [DOI] [PubMed] [Google Scholar]
- 16.Chang HJ, Kim MH, Baek MK, Park JS, Chung IJ, Shin BA, et al. Triptolide inhibits tumor promoter-induced uPAR expression via blocking NF-κB signaling in human gastric AGS cells. Anticancer research. 2007;27(5A):3411–7. [PubMed] [Google Scholar]
- 17.Chong EWT, Sinclair AJ, Guymer RH. Facts on fats. Clinical & experimental ophthalmology. 2006;34(5):464–71. [DOI] [PubMed] [Google Scholar]
- 18.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21(6):495–505. [DOI] [PubMed] [Google Scholar]
- 19.Whyte C, Thies F, Peyrol L, Balcerzak D. N-3 long-chain polyunsaturated fatty acids inhibit smooth muscle cell migration by modulating urokinase plasminogen activator receptor through MEK/ERK-dependent and-independent mechanisms. The Journal of nutritional biochemistry. 2012;23(11):1378–83. 10.1016/j.jnutbio.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 20.Chen H-W, Chao C-Y, Lin L-L, Lu C-Y, Liu K-L, Lii C-K, et al. Inhibition of matrix metalloproteinase-9 expression by docosahexaenoic acid mediated by heme oxygenase 1 in 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 human breast cancer cells. Archives of toxicology. 2013;87(5):857–69. 10.1007/s00204-012-1003-3 [DOI] [PubMed] [Google Scholar]
- 21.Fang IM, Yang CH, Yang CM. Docosahexaenoic acid reduces linoleic acid induced monocyte chemoattractant protein-1 expression via PPARγ and nuclear factor-κB pathway in retinal pigment epithelial cells. Molecular nutrition & food research. 2014;58(10):2053–65. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Analytical biochemistry. 2000;278(2):175–84. [DOI] [PubMed] [Google Scholar]
- 23.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cδ/c-Src pathways in glioblastoma cells. Journal of Biological Chemistry. 2005;280(9):7729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews CP, Colburn NH, Young MR. AP-1 a target for cancer prevention. Current cancer drug targets. 2007;7(4):317–24. [DOI] [PubMed] [Google Scholar]
- 25.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87(1):13–20. [DOI] [PubMed] [Google Scholar]
- 26.Cheng CY, Kuo CT, Lin CC, Hsieh HL, Yang CM. IL-1β induces expression of matrix metalloproteinase-9 and cell migration via ac-Src-dependent, growth factor receptor transactivation in A549 cells. British journal of pharmacology. 2010;160(7):1595–610. 10.1111/j.1476-5381.2010.00858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, et al. Global cancer facts & figures 2007. Atlanta, GA: American Cancer Society; 2007;1(3):52. [Google Scholar]
- 28.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. Journal of Biological Chemistry. 2002;277(11):8755–8. [DOI] [PubMed] [Google Scholar]
- 29.Ye S, Tan L, Ma J, Shi Q, Li J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins, Leukotrienes and essential fatty acids. 2010;83(1):37–43. [DOI] [PubMed] [Google Scholar]
- 30.Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proceedings of the National Academy of Sciences. 2013;110(12):4816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Current opinion in lipidology. 2008;19(3):242 10.1097/MOL.0b013e3282ffaf6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song N-Y, Na H-K, Baek J-H, Surh Y-J. Docosahexaenoic acid inhibits insulin-induced activation of sterol regulatory-element binding protein 1 and cyclooxygenase-2 expression through upregulation of SIRT1 in human colon epithelial cells. Biochemical pharmacology. 2014;92(1):142–8. 10.1016/j.bcp.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 33.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, et al. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and-2 and HIF-1α induction pathway. Carcinogenesis. 2004;25(12):2303–10. [DOI] [PubMed] [Google Scholar]
- 34.Chao C-Y, Lii C-K, Ye S-Y, Li C-C, Lu C-Y, Lin A-H, et al. Docosahexaenoic acid inhibits vascular endothelial growth factor (VEGF)-induced cell migration via the GPR120/PP2A/ERK1/2/eNOS signaling pathway in human umbilical vein endothelial cells. Journal of agricultural and food chemistry. 2014;62(18):4152–8. 10.1021/jf5007165 [DOI] [PubMed] [Google Scholar]
- 35.Cho Y, Turner N, Davidson L, Chapkin R, Carroll R, Lupton J. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Experimental biology and medicine (Maywood, NJ). 2014;239(3):302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–6. 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- 37.Tan M, Li P, Sun M, Yin G, Yu D. Upregulation and activation of PKCα by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKCα and Src inhibitors. Oncogene. 2006;25(23):3286–95. [DOI] [PubMed] [Google Scholar]
- 38.Beit-Yannai E, Shmulevich A. Does the aqueous humor have a role in mitogen-activated protein kinase (MAPK) intracellular signaling in Glaucoma? Medical hypotheses. 2007;68(2):299–302. [DOI] [PubMed] [Google Scholar]
- 39.Park H, Chang SK, Kim JY, Lee BM, Shin HS. Risk Factors for Distant Metastasis as a Primary Site of Treatment Failure in Early-Stage Breast Cancer. Chonnam medical journal. 2014;50(3):96–101. 10.4068/cmj.2014.50.3.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Ghiso JAA, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1(5):445–57. [DOI] [PubMed] [Google Scholar]
- 41.Ashour AA, Gurbuz N, Alpay SN, Abdel-Aziz AAH, Mansour AM, Huo L, et al. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial–mesenchymal transition mediating pancreatic cancer cells invasion. Journal of cellular and molecular medicine. 2014;18(11):2235–51. 10.1111/jcmm.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resh MD. The ups and downs of SRC regulation: tumor suppression by Cbp. Cancer Cell. 2008;13(6):469–71. 10.1016/j.ccr.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Park JH, Khoi PN, Joo YE, Do Jung Y. MSP-induced RON activation upregulates uPAR expression and cell invasiveness via MAPK, AP-1 and NF-κB signals in gastric cancer cells. Carcinogenesis. 2011;32(2):175–81. 10.1093/carcin/bgq241 [DOI] [PubMed] [Google Scholar]
- 44.Estève PO, Chicoine É, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, et al. Protein kinase C-ζ regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-α in glioma cells via NF-κB. Journal of Biological Chemistry. 2002;277(38):35150–5. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011; 1: 524–38. CD-11-0124.[PMC free article][PubMed][Cross Ref]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ, Kim NH, et al. Cadmium induces matrix metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and AP-1 pathways in human endothelial cells. Toxicology. 2015;338:104–16. [DOI] [PubMed] [Google Scholar]
- 47.Morgan MJ, Liu Z-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell research. 2011;21(1):103–15. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zannetti A, Del Vecchio S, Carriero MV, Fonti R, Franco P, Botti G, et al. Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer research. 2000;60(6):1546–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.