Figure 1.
A Single-Molecule FRET Assay for Real-Time Initial Transcription
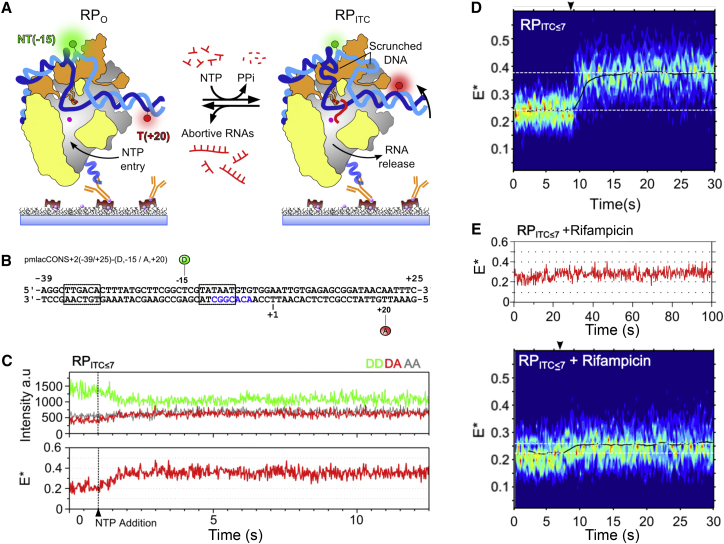
(A) Schematic of assay. Left, RPo; right, initial transcribing complex (ITC). Donor is in green; acceptor in red; σ70 in orange; RNAP in gray, except for the β subunit (omitted for clarity) and regions protruding from the cut-away plane (in yellow); template strand in blue; non-template strand in teal; nascent RNA in red; and RNAP active site in pink. The penta-His antibody anchors RPo to the surface. The initial FRET efficiency is low; upon NTP addition, scrunching moves the acceptor closer to the donor, increasing FRET efficiency.
(B) lacCONS DNA fragment for FRET assay; the −10/−4 pre-melted region is in blue.
(C) Time trace showing an increase to E∗∼0.37 upon adding 80 μM UTP and GTP to form RPITC≤7. The NTP addition point is marked with a dashed line. Frame time: 20 ms. DD trace (green trace, top), donor emission upon donor excitation; DA trace (red trace, top), acceptor emission upon donor excitation; AA trace (gray trace, top), acceptor emission upon acceptor excitation. DD and DA are used for calculating apparent FRET efficiency E∗.
(D) Transcription heatmaps (n = 45) showing activity upon NTP addition to form RPITC≤7. NTP addition is marked by an arrowhead. Blue to red colors represent an increasing number of events. Black line, time trace of average E∗ of all traces; white dotted lines, E∗ for RPo baseline (at E∗∼0.24) and RPITC plateau (at E∗∼0.37). Frame time: 200 ms.
(E) Time trace (top) and transcription heatmap (bottom, n = 37) for RPITC≤7 in the presence of rifampicin.
See also Figure S1.