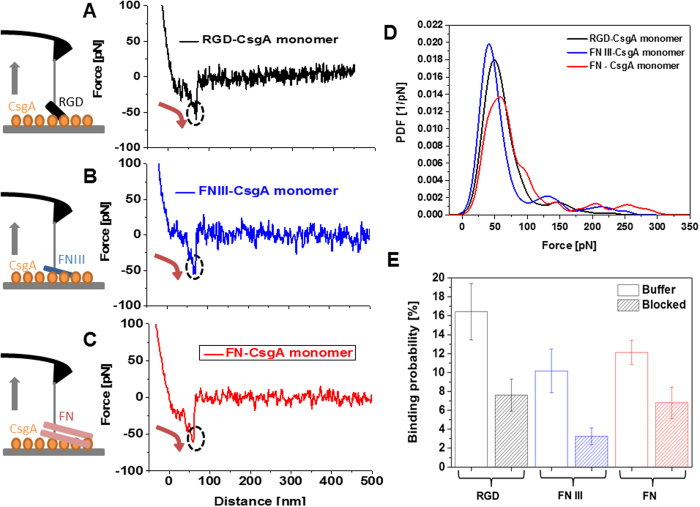
Figure 1. Single-molecular force spectroscopy experiments on surface-bound CsgA monomers.
Typical force-distance curves recorded using AFM cantilever tips functionalized with (A) RGD. (B) FN III. (C), FN. Monomeric CsgA was tethered to silicon chip surfaces (sketches in (A–C)) via a flexible poly(ethylene glycol) (PEG) chain. Likewise, the RGD peptide, FN III, or FN were flexibly linked to AFM tips. The PEG linker that connected the molecules to the AFM tips and probe surfaces (Fig. S1) ensured sufficient motional freedom for unconstrained interaction measurements. Sketches on the left side show bond formation and the downward deflection of the AFM cantilever during retraction. Red arrows in force-distance curves indicate a bond load increase, circles mark bond rupture. (D) PDFs of unbinding forces at a retraction velocity of 500 nm/s. For each PDF 1000 force curve measurements were recorded. (E) Binding probability (defined as the percentage of force experiments displaying unbinding events) for RGD (n = 3000, 3 different tips), FN III (n = 3000, 3 different tips), and FN. (n = 6000, 6 different tips) Addition of RGD peptides into the measurement solution (blocked) resulted in a significant drop of the binding probability: from 16 to 7% for RGD (n = 3000, 3 tips), from 10 to 3% for FN III (n = 3000, 3 tips), and from 12 to 6% for FN (n = 3000, 3 tips).