Abstract
Background
Head and neck squamous cell carcinomas (HNSCC) are known to evade the host immune response. How premalignant oral lesions modulate the immune response, however, has yet to be elucidated.
Materials and Methods
A mouse model of oral carcinogenesis was used to determine how mediators from premalignant oral lesion cells vs. HNSCC cells impact on immune cytokine production and activation.
Results
Media conditioned by premalignant lesion cells elicited an increased production of T cell-associated cytokines and proinflammatory mediators from cervical lymph node cells compared to media conditioned by HNSCC cells or media alone. In the presence of premalignant lesion cell-conditioned media, CD4+ T cell expression of the IL-2 receptor CD25 and CD8+ T cell expression of the activation marker CD69 was greater, compared to what was induced in HNSCC cell-conditioned media or media alone.
Conclusion
Premalignant lesion cells promote a proinflammatory environment and induce immune changes before HNSCC tumors are established.
Keywords: head and neck cancer, premalignant oral lesions, cytokines, T cell activation
Head and neck squamous cell carcinoma (HNSCC) is an aggressive malignancy with a survival rate that remains approximately 50% (1, 2). One of the most challenging obstacles to treatment is that patients typically present with advanced tumors, that have adapted mechanisms to evade the host immune response (1, 3–5). These advanced tumors are also characterized by high recurrence rates, contributing to the mortality of the disease (6, 7).
HNSCC patients are characterized by immunosuppression, both systemically and at the site of the tumor. Increased levels of circulating T regulatory cells and CD34+ progenitor cells have been shown to suppress CD8+ T cell-mediated immunity and are associated with a poorer prognosis (8–11). There is also evidence to show a systemic Th2-skewing of T cells, as peripheral blood mononuclear leukocytes (PBMLs) isolated from HNSCC patients secrete abnormally high levels of Th2-type cytokines, that are less effective against tumors compared to Th1-type cytokines, including interferon-γ (IFN-γ) (12, 13). Several studies have shown that HNSCC tumors themselves set up an immunosuppressive environment to thwart the host immune response. HNSCC cells are characterized by decreased major histocompatibility complex (MHC) and costimulatory molecule expression, increased FasL expression and direct secretion of immunosuppressive molecules such as transforming growth factor-β (TGF-β) and prostaglandin E2 (PGE2) (3, 5, 14–19). As a result, tumor-infiltrating CD8+ T cells exhibit a number of functional defects and are characterized by decreased IL-2 expression and decreased proliferation, which can also contribute to persistence of tumor (3, 11, 18, 20, 21). HNSCC tumors also promote the infiltration of immunosppressive T regulatory cells and myeloid-derived suppressor cells (MDSCs), which are associated with a worse prognosis and increased metastasis in patients (8–10, 16, 22–24).
While a few studies have investigated how established HNSCC tumors evade the host immune response, there remains a critical gap in knowledge of how premalignant lesions modulate the immune response and how this impacts on progression to tumor. A few studies examining the immune environment of premalignant oral lesions in patients have shown that premalignant tissue is characterized by increased levels of proinflammatory mediators, including IFN-γ, IL-6 and IL 17A, compared to HNSCC tumor tissue (25). Several studies have also shown that saliva of patients bearing premalignant oral lesions contains increased levels of proinflammatory IL-6 and tumor necrosis factor-α (TNF-α), suggesting that a local inflammatory response occurs in the oral cavities of patients with premalignant lesions (26, 27). There is also evidence that premalignant lesions are characterized by an influx of proinflammatory immune cells, including NK cells, macrophages, and CD8+ T cells and that CD8+ T cells are more activated in premalignant oral tissue (28–30). While these studies offer some insight into the immune environment of premalignant oral lesions, they are based on a low number of patients and do not delve into the mechanism by which mediators released by the lesions themselves impact on immune cell reactivity.
The current study is based on the 4-NQO mouse model of oral carcinogenesis, that delivers carcinogenic 4 NQO to C57BL/6 mice via drinking water. This model mimics the effects of tobacco and induces molecular and genetic changes similar to those that occur in the development of HNSCC in patients (31–37). Most importantly, this model allows careful investigation into the immune changes associated with progression of premalignant lesions to HNSCC, which is not possible with other models including models in which tumor cells are injected either subcutaneously or orthotopically into mice. Previous studies using the 4-NQO model have shown that T cells isolated from the cervical lymph nodes of premalignant lesion-bearing mice are characterized by increased expression of activation markers, such as CD69, and increased secretion of IL-2 compared to T cells isolated from HNSCC-bearing mice (38). Furthermore, cervical lymph nodes of premalignant lesion-bearing mice are characterized by an increased population of proinflammatory Th17 cells and increased levels of proinflammatory cytokines, including IL 17A, compared to cervical lymph nodes of HNSCC-bearing mice (38). These studies suggest that an active immune response is occurring at the preneoplastic stage, but once the tumor becomes established, the response is significantly dampened. How the premalignant lesion vs. HNSCC tumor modulates immune reactivity at the site of the developing lesion or the tumor has not been extensively explored.
Progressive development of cancer affects multiple cell types which, in turn, can have immune impact. Thus, the present study aimed to advance our original findings by dissecting the direct contribution of the premalignant lesion and oral cancer cells on the immune stimulatory condition in mice with premalignant oral lesions. To address this question, primary cell cultures were established from mice bearing premalignant lesions and HNSCC tumors, and supernatants were collected for culture with cervical lymph node cells or spleen cells from healthy control C57BL/6 mice. The results of the current study show that cervical lymph node cells produce increased levels of Th1-, Th2- and Th17-associated cytokines, as well as proinflammatory mediators, in the presence of media conditioned by premalignant lesion cells compared to media conditioned by HNSCC cells. Flow cytometric analysis showed that an increased percentage of both CD4+ and CD8+ T cells expressed IFN-γ in the presence of media conditioned by premalignant lesion cells compared to expression in T cells in the presence of media conditioned by HNSCC cells. Analysis of surface marker expression showed that, in the presence of media conditioned by premalignant lesion cells, an increased percentage of CD4+ T cells expressed CD25 and an increased percentage of CD8+ T cells expressed CD69. A striking population of CD69hi-expressing cells was observed in both the CD4+ and CD8+ T cell compartments in the presence of premalignant lesion cell supernatant. These data support the hypothesis that the premalignant lesion environment is significantly more immune stimulatory compared to the HNSCC environment, eliciting increased cytokine production and increased expression of activation markers on T cells.
Materials and Methods
Oral carcinogenesis model
To establish premalignant oral lesions that progress to HNSCC, female C57BL/6 mice (Charles Rivers Laboratory, Wilmington, MA, USA) were administered 50 μg/ml 4-NQO in their drinking water starting at 2 months of age (38). At the molecular level, 4-NQO causes the formation of reactive oxygen species (ROS), DNA adducts (adenosine to guanosine substitutions) and H-ras mutations, mimicking the DNA damage induced by tobacco and alcohol use, and therefore modeling human disease at the molecular level 33, 34). In this model, premalignant oral lesions develop at 6–8 weeks and HNSCC appears at 12–16 weeks of 4 NQO treatment. At weekly intervals, mice were examined endoscopically for lesion development. This was performed with a Stryker 1.9 mm × 30° endoscope, with images being taken with a Stryker 1088 camera (Kalamazoo, MI, USA) (39). Also, histological analysis of tongue tissue from 4NQO-treated mice was performed by oral pathology in the Center for Oral Health Research within the College of Dental Medicine at the Medical University of South Carolina. These histological analyses, that were performed in a blinded manner, revealed a progressive increase in the overall degree of dysplasia in tongue tissue, which coincided with endoscopic detection of premalignant lesions on the tongue. All animal studies were approved by the Institutional Animal Care and Use Committee at the Ralph H. Johnson Veterans Affairs Medical Center.
Culture medium
Cell culture media consisted of 1× DMEM (Life Technologies, Grand Island, NY, USA) containing 4.5 g/l D-glucose and L-glutamine, supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA) and 1× antibiotic antimycotic solution (Sigma, St. Louis, MO, USA) containing penicillin, streptomycin and amphotericin B. To establish cell lines from premalignant lesions and HNSCC tumors, culture media was supplemented with 2× antibiotic antimycotic solution for the first 2 weeks of culture.
Premalignant lesion and HNSCC cell lines
Primary cell lines were established as previously described by excising the tongues of premalignant or HNSCC-bearing mice at the appropriate stage, as defined through histopathological analysis by the oral pathology section at the Medical University of South Carolina (39). Briefly, excised premalignant lesions or HNSCC tumors were placed into culture and, once adherent cells became established, non-adherent cells were removed. Media were replaced during establishment of lesions cells or HNSCC cells twice weekly. Once the cells’ epithelial phenotype was determined by positive E-cadherin staining and uniformity of their microscopic and growth characteristics was also confirmed, 48 h supernatants from sub-confluent cultures (80%) were collected for use in specific analyses described below.
Cervical lymph node processing
Cervical lymph nodes were harvested from control C57BL/6 mice and homogenized using a Stomacher 80 homogenizer (Seward) set on high for 90 sec. Cells were then passed through a 70-μm cell strainer (BD Falcon, San Jose, CA, USA) and rinsed with HBSS. Cell number was determined by counting cells excluding trypan blue using a hemocytometer.
Spleen processing
Spleens were harvested from control C57BL/6 mice and homogenized using a glass homogenizer. Cells were passed through a 70-μm cell strainer (BD Falcon, San Jose, CA, USA) and rinsed with HBSS. Red blood cells were lysed by adding ACK Lysing Buffer (Lonza, Walkersville, MD, USA) for 3 min. Splenocytes were then washed twice with HBSS. Cell number was determined by counting cells excluding trypan blue using a hemocytometer.
Cervical lymph node cell cultures
Cervical lymph node cells were cultured in 12-well anti-CD3-coated tissue culture plates at 1×106 cells/well in fresh media or media conditioned by premalignant lesions or HNSCC, with IL-2 (R&D Systems, Minneapolis, MN, USA) for 72 h at 37°C. Premalignant lesion cells and HNSCC cell supernatants were diluted 1:2 in fresh media for culture with lymph node cells. For cytokine analysis, cells were re-stimulated with 2 μl/ml Cell Stimulation Cocktail (eBioscience, PMA/ionomycin) for the last 4–6 h of culture at 37°C and supernatants were collected for analysis.
Cytokine bead array
All reagents used for the cytokine bead array are from BD Biosciences (San Jose, CA, USA). The levels of IFN-γ, IL-2, IL-17A, IL-4, IL-6 and IL-10 in cell culture supernatants were determined using a mouse cytometric bead array (CBA) Th1/Th2/Th17 cytokine kit. Levels of IL-1α, IL-1β, IL 13, IL-12p70, IL-9, GM-CSF, G-CSF, MIG, MCP-1, MIP-1α, MIP-1β and RANTES in cell cultures were determined using cytometric bead array flex sets for the individual cytokines according to the manufacturer’s instructions. A FACS Canto (BD Biosciences) flow cytometer was used to quantify cytokine profiles and relative amounts of each cytokine were analyzed using FCAP Array Software (manufactured by Soft Flow Hungary Ltd. for BD Biosciences). Cytokine levels in premalignant lesion cell and HNSCC cell supernatants were measured before addition to cultures with cervical lymph node cells or spleen cells, respectively, and these levels were subtracted out of the total cytokine levels in these cell cultures analyses.
Spleen cell phenotypic and cytokine analyses
All antibodies and reagents for this section were from BDBiosciences (San Jose, CA, USA) unless otherwise stated. Spleen cells were cultured in 12-well anti-CD3-coated tissue culture plates at 1×106 cells/well in fresh media or in a 1:2 dilution of media conditioned by premalignant lesion cells or HNSCC cells with IL-2 (R&D Systems, Minneapolis, MN, USA) for 72 h at 37°C. For analysis of intracellular cytokine expression by flow cytometry, cells were re-stimulated with 2 μl/ml Cell Stimulation Cocktail (eBioscience, PMA/ionomycin) and 0.6 μl/ml GolgiStop for the last 4–6 h of culture at 37°C. Cells were collected and washed once with stain buffer, and resuspended in 300 μl FACS block buffer, containing 2% FBS in sterile 1× PBS, at 4°C for 15 min. They were then incubated in 10 μl Fc block, containing anti CD16/32 antibody at a 1:100 dilution in sterile 1× PBS, at 4°C for 10 min to block nonspecific antibody binding. For surface staining, cells were then stained with equal concentrations of the following antibodies or appropriate isotype controls: FITC CD4, PE/APC CD8a, PE CD69 and APC CD25 (eBioscience). Cells were washed twice and resuspended in 400 μl stain buffer for analysis using a FACS Canto flow cytometer. For intracellular staining, cells were then washed twice in stain buffer, resuspended in 1 mL cold Cytofix for 20 min at 4°C. After washing, cells were resuspended in 1 ml Perm/Wash Buffer for 15 min at room temperature, washed and resuspended in 50 μl Perm/Wash Buffer with equal concentrations of the following antibodies or appropriate isotype controls: FITC CD4, APC/PE CD8a and PerCP-Cy5.5 IFN γ. After 30 min, cells were washed and analyzed on a FACS Canto flow cytometer.
Statistical analyses
Data were reported as means±standard error of the mean. To compare one variable condition between groups, a one-way ANOVA analysis was initially performed (GraphPad Prism version 6.03 for Windows, GraphPad Software, La Jolla, CA, USA). Any differences that were identified by ANOVA analysis were further analyzed by a two-tailed Student’s t-test between each of two groups. Significance was reported in the 95% confidence interval.
Results
Premalignant lesion cell-conditioned media elicit increased production of Th1-, Th2- and Th17-associated cytokines from cervical lymph node cells
Previous studies have suggested that the premalignant lesion environment is more immune stimulatory than the established tumor environment (39). To investigate the effect of the premalignant lesion vs. HNSCC environments on cytokine production by immune cells in the cervical lymph nodes, cervical lymph node cells from control C57BL/6 mice were cultured with media conditioned by premalignant lesion cells, HNSCC cells, or media alone and the levels of secreted Th1-, Th2- and Th17-associated cytokines and other pro-inflammatory mediators were analyzed by cytokine bead array.
As shown in Figure 1, cervical lymph node cells secreted increased quantities of Th1-associated IL 2, IFN-γ and TNF-α in the presence of media conditioned by premalignant lesion cells compared to HNSCC cells or media alone, suggesting that the premalignant lesion environment induces a more activated Th1-type response. The levels of these cytokines produced by lymph node cells in the presence of HNSCC cell-conditioned media were increased compared to the levels produced in media alone, demonstrating that factors released by the tumor elicit a slightly increased Th1-type response. Even still, this response is much lower in magnitude compared to the response elicited by the premalignant lesion environment. Cervical lymph node cells also produced increased levels of Th2-associated and inflammatory cytokines, including IL 4, IL-6 and IL-17A in the presence of premalignant lesion cell-conditioned media compared to levels produced in HNSCC cell-conditioned media or media alone, suggesting that the premalignant lesion environment may stimulate both Th1- and Th2-type responses. Cervical lymph node cells also produced increased levels of several proinflammatory mediators associated with macrophage activation and function, including MIP-1α, MIP-1β and G-CSF, in the presence of premalignant lesion cell-conditioned media compared to levels produced in HNSCC cell-conditioned media or media alone.
Figure 1.
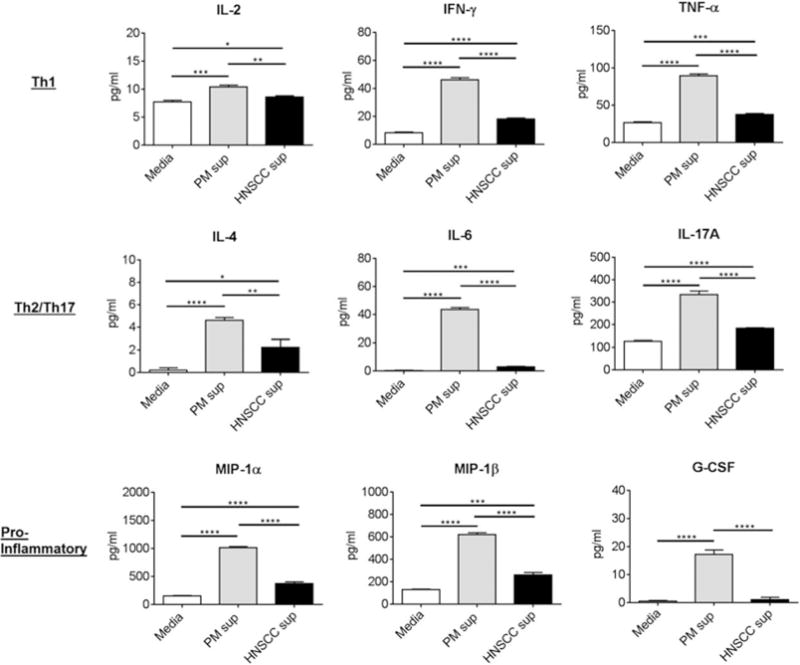
Cervical lymph node cells release increased levels of Th1-, Th2- and Th17-associated cytokines and proinflammatory mediators in the presence of premalignant cell-conditioned media compared to levels released by lymph node cells in the presence of HNSCC cell-conditioned media or fresh media alone. Cervical lymph node cells were extracted from control C57BL/6 mice and cultured with premalignant lesion cell conditioned media, HNSCC cell-conditioned media or media alone for 72 h. Cell cultures were restimulated with PMA/ionomycin for the last 4 h of culture and supernatants were collected for cytokine analyses by CBA. Data shown represent mean±SEM of three independent experiments, each performed in duplicate. *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001 (two tailed Student’s t-test).
These data suggest that factors released by premalignant lesion cells stimulate the production of innate proinflammatory mediators by immune cells, in addition to stimulating cytokine production by T cells. These data show that the environment set up by the premalignant lesion cells themselves stimulates the production of a spectrum of inflammatory mediators and cytokines by immune cells in the cervical lymph nodes and suggests that premalignant lesion cells may be modulating the reactivity of immune cells at the site of the developing lesion.
Increased percentage of splenic CD4+ and CD8+ T cells express IFN-γ in the presence of premalignant lesion cell-conditioned media
The above studies demonstrate that mediators released by premalignant lesion cells elicit increased Th1-, Th2-, and Th17-associated cytokine production by immune cells in the cervical lymph nodes compared to mediators released by HNSCC cells. These studies suggest that the premalignant lesion environment is more inflammatory than the HNSCC environment, but the effect of the premalignant lesion and HNSCC environments on CD4+ vs. CD8+ T cell cytokine production, specifically, has yet to be fully elucidated.
To investigate whether factors produced by premalignant lesion cells elicit increased expression of the Th1-type cytokine IFN-γ, spleen cells from control C57/BL6 mice were cultured with media conditioned by premalignant lesion cells or HNSCC cells and analyzed by flow cytometric. Also shown in Figure 2 is an increased percentage of CD4+ T cells expressed IFN-γ in the premalignant lesion environment compared to CD4+ T cells in the HNSCC environment or media alone. This trend was more striking with CD8+ T cells, as premalignant lesion cell-conditioned media elicited significantly increased numbers of IFN-γ-expressing CD8+ T cells compared to the effect of HNSCC cell-conditioned media or media alone. These data demonstrate that mediators released by premalignant lesion cells elicit increased expression of the Th1-type cytokine IFN-γ in CD4+ and, to a more prominent extent in CD8+ T cells, and suggest that the premalignant lesion environment supports a more robust Th1-type response compared to the HNSCC environment or media alone.
Figure 2.
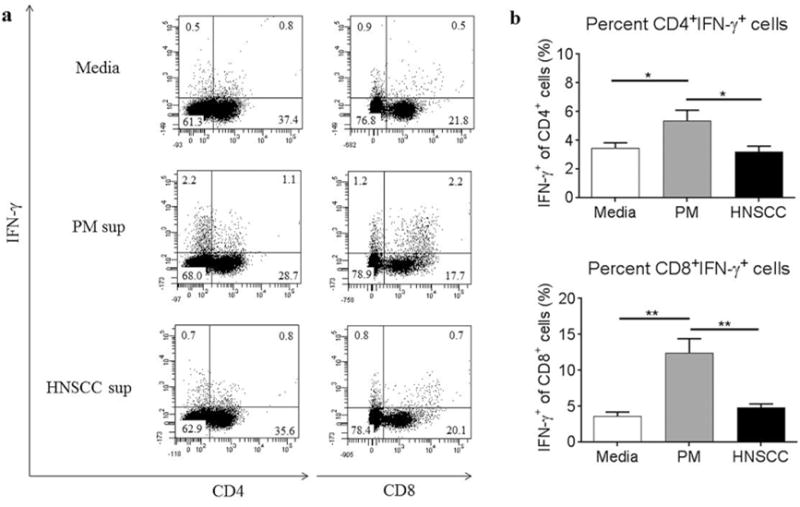
Premalignant lesion cell-conditioned media elicits increased expression of IFN-γ in CD4+ and CD8+ splenic T cells compared to cytokines produced in HNSCC cell conditioned media or media alone. Representative results (a) and graphical representation (b) of flow cytometric staining of spleen cells from control C57BL/6 mice cultured with media alone, premalignant lesion cell-conditioned media, or HNSCC cell-conditioned media for 72 h. Spleen cells were restimulated with PMA/ionomycin for the last 4 h of culture. Data show staining of spleen cells from 3 independent experiments, run in duplicate. Data represent mean±SEM. *p<0.05 **p<0.01 (two-tailed Student’s t-test).
Increased percentage of CD4+ T cells express CD25 in the presence of premalignant lesion cell-conditioned media
The impact of the premalignant lesion environment on the activation status of T cells, a critical component of T cell function and persistence in the developing lesion/tumor environment, has not been extensively explored. One previous study in the 4-NQO model showed that a greater percentage of conventional CD4+ T cells in the cervical lymph nodes of premalignant lesion-bearing mice expressed CD25 compared to CD4+ T cells isolated from HNSCC-bearing or control mice, suggesting that T cells are more activated in premalignant lesion-bearing mice (38). However, the direct impact of the premalignant lesion and HNSCC environments, i.e. factors produced by the premalignant lesion cells and HNSCC cells themselves, on the expression of CD25, has not been investigated.
To investigate the impact of the premalignant lesion and tumor environments on CD25 expression in T cells, spleen cells from control C57BL/6 mice were cultured with premalignant lesion cell-conditioned media, HNSCC cell-conditioned media or media alone and then analyzed by flow cytometric staining. Lymphocytes were gated as shown in Figure 2. As shown in Figure 3, a greater percentage of CD4+ T cells expressed CD25 in the presence of premalignant lesion cell-conditioned media compared to HNSCC cell-conditioned media or media alone, suggesting that the premalignant lesion cells elicit increased activation of CD4+ T cells compared to tumor cells. The MFI of CD25 staining for CD4+ T cells in the premalignant lesion environment (1,899) was also increased, although not to a significant degree, compared to those that were in the HNSCC environment (1,387). In the CD8+ T cell compartment, premalignant lesion cell-conditioned media elicited an increased percentage of CD25+ cells compared to media alone, although the results were not significant. However, the MFI of CD25 staining for CD8+ T cells in the premalignant lesion environment (1,933) was increased compared to those in media alone (775). There was no difference in the percentage of CD8+ T cells expressing CD25 in the premalignant lesion vs. HNSCC environments, although the MFI of CD25 staining for CD8+ T cells was increased in the premalignant lesion environment (1,933) compared to the HNSCC environment (1,054). Overall, the results of these studies demonstrate that soluble mediators released by premalignant lesion cells elicit an increase in a population of CD25-expressing CD4+ T cells, suggesting that the premalignant lesion environment is more immune stimulatory than the HNSCC environment.
Figure 3.
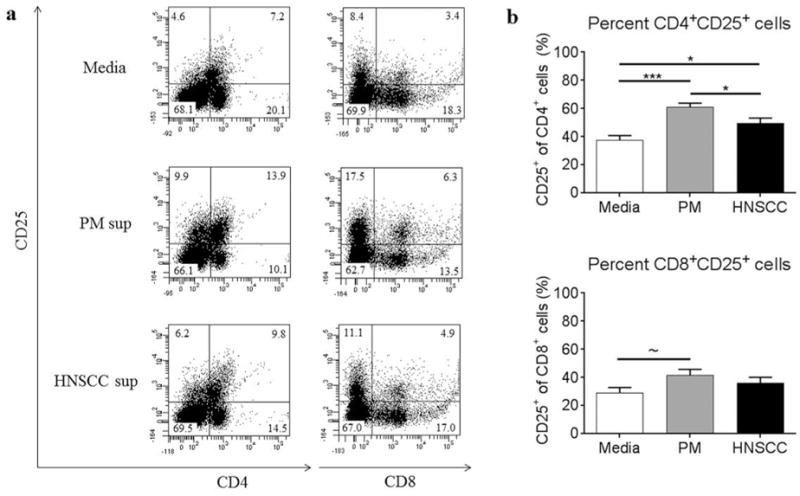
Premalignant lesion cell-conditioned media elicits increased expression of CD25 on CD4+ T cells compared to HNSCC cell-conditioned media or media alone. Representative results (a) and graphical representation (b) of flow cytometric staining of spleen cells from control C57BL/6 mice cultured with media alone, premalignant lesion cell conditioned media, or HNSCC cell-conditioned media for 72 h. Data show staining of spleen cells from 3 independent experiments, run in duplicate. Data represent mean±SEM. *p<0.05 **p<0.01 ***p<0.001 ~p=0.056 (two-tailed Student’s t-test).
Premalignant lesion cell-conditioned media elicits significantly increased expression of CD69 in a subset of CD4+ and CD8+ T cells
The activation and persistence of T cells in the developing lesion and tumor environments is dependent on a number of factors, including the expression of the early activation marker CD69. CD69 is involved in T cell proliferation and function and therefore serves as an important marker of T cell stimulation.
To investigate the impact of the premalignant lesion environment on CD69 expression on T cells, spleen cells from control C57BL/6 mice were cultured with premalignant lesion cell-conditioned media, HNSCC cell-conditioned media, or media alone and cells were then analyzed by flow cytometric staining. Lymphocytes were gated as shown in Figure 2. As shown in Figure 4b, the overall percentage of CD4+ T cells expressing CD69 in the presence of HNSCC cell-conditioned media was decreased compared to T cells in the presence of media alone. This suggests that CD4+ T cells in the tumor environment are less stimulated compared to control. However, there was no difference in the percentage of CD4+ T cells expressing CD69 in the presence of premalignant lesion cell-conditioned media compared to T cells in the presence of HNSCC cell-conditioned media. The overall percentage of CD8+ T cells expressing CD69 in the presence of premalignant lesion cell-conditioned media, however, was increased compared to T cells in the presence of HNSCC cell-conditioned media or media alone. This suggests that the premalignant lesion environment stimulates increased CD69 expression selectively on CD8+ T cells. Furthermore, as gated in Figure 4a, we observed an increased population of CD69hi-expressing cells in both the CD4+ and CD8+ T cell compartments in the presence of premalignant lesion cell-conditioned media compared to T cell populations in the presence of HNSCC cell-conditioned media or media alone (Figure 4c). These data show that the premalignant lesion environment elicits significantly increased expression of CD69 on a subset of both CD4+ and CD8+ T cells compared to the tumor environment and suggest that the premalignant lesion environment is more immune stimulatory.
Figure 4.
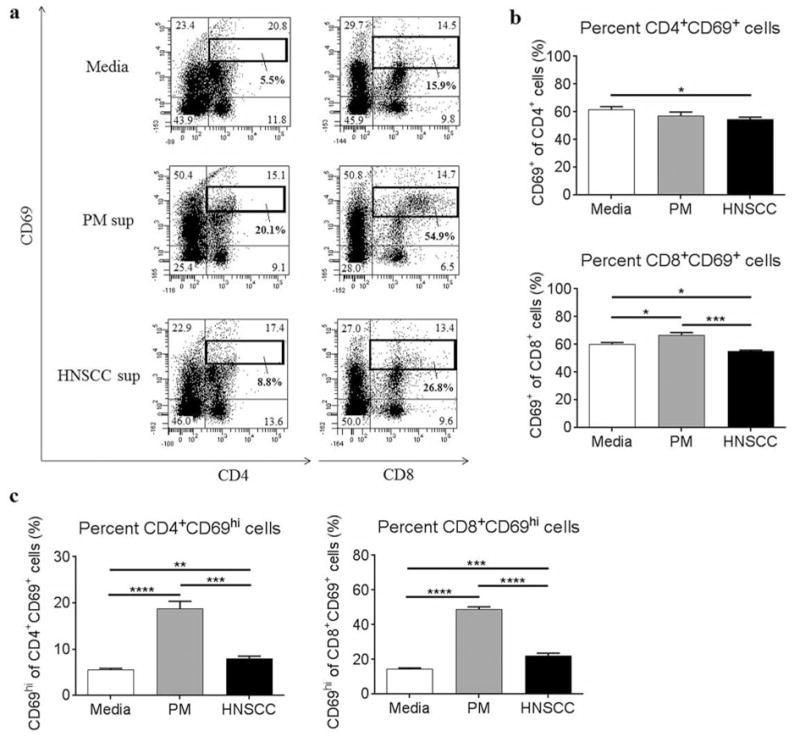
Premalignant lesion cell-conditioned media elicits increased expression of CD69 on CD4+ and CD8+ T cells, compared to HNSCC cell-conditioned media or media alone. Representative results (a) and graphical representation (b) of flow cytometric staining of spleen cells from control C57BL/6 mice cultured with media alone, premalignant lesion cell conditioned media, or HNSCC cell-conditioned media for 72 h. Data show staining of spleen cells from 3 independent experiments, run in duplicate. Graphical results of the percentage of CD69hi populations among CD4+CD69+ cells and CD8+CD69+ cells gated in (a) are shown in (c). Data represent mean±SEM. *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001 (two-tailed Student’s t-test).
Discussion
Although the immunosuppressive environment of established HNSCC tumors has been well studied, less focus has been given to what the present study has shown to be a stage in which an inflammatory immune response is active, and yet unsuccessful at eliminating, the dysplastic tissue, i.e. the premalignant lesion stage.
The first goal of the current study was to determine how immune mediators released by premalignant lesion cells modulate cytokine production by immune cells in the cervical lymph nodes, which drain the developing lesion/tumor. Previously, our lab has shown that premalignant lesion cells isolated from mice with 4-NQO-induced premalignant lesions release a panel of immune modulators, including MCP-1, RANTES, G-CSF, and PGE2, suggesting a role for premalignant lesion cells as modulators of the local immune response (39). The current study shows that in the presence of media conditioned by premalignant lesion cells, cervical lymph node cells produce increased levels of Th1-, Th2- and Th17-associated cytokines, as well as several proinflammatory mediators, including granulocyte macrophage-colony stimulating factor (GM-CSF) and G-CSF. Taken together, these data show that mediators released directly by premalignant lesion cells elicit increased production of inflammatory cytokines by immune cells and suggest that premalignant lesions set up a cycle of inflammation at the site of the developing lesion. Interestingly, these studies show that factors released by premalignant lesion cells elicit increased production of both Th1-type cytokines, including IL-2 and IFN-γ, as well as Th2-type cytokines, including IL-4, and therefore cannot be classified singularly as ‘pro-Th1.’ It may be that factors released by premalignant lesion cells elicit cytokine production from both Th1- and Th2-type cells, or the increase in Th2-type cytokines may be the response to an initial dramatic increase in proinflammatory Th1-type cytokines, as a mechanism to turn off the inflammation. In this way, the premalignant lesion state could represent a battle of immune cells secreting proinflammatory mediators to eliminate the dysplastic tissue against immunosuppressive cells secreting immune inhibitory molecules to dampen inflammation, which ultimately can allow the establishment of HNSCC. As the current study demonstrates, factors released by HNSCC cells do not elicit a significant Th1- or Th2-type response from immune cells because by the time the tumor is established, the battle has essentially been lost. Several of the cytokines presented, including IL-2, play critical roles in the differentiation of T cells as well as activation of effector T cells. However, the plasticity of T cells necessitates that the cytokine phenotype of T cells should be viewed as a snapshot, and as shown in our own studies, varies depending on the mediators that are produced by non-immune cells such as premalignant lesion cells or HNSCC cells.
To further analyze the effect of the premalignant lesion environment on cytokine expression by different subsets of T cells, spleen cells from control C57BL/6 mice were cultured with media conditioned by premalignant lesion cells vs. HNSCC cells or media alone. Analysis of intracellular expression of IFN-γ by T cells showed that an increased percentage of both CD4+ and CD8+ T cells express IFN-γ in the presence of media conditioned by premalignant lesion cells compared to media conditioned by HNSCC cells or media alone. These data show that mediators released directly by premalignant lesion cells elicit increased IFN-γ expression in T cells and suggest that the premalignant lesion environment supports an increased Th1-type response. Future studies focusing on flow cytometric analysis of total numbers of Th1 /Th2- type cells and intracellular expression of other cytokines, including TNF α, IL-4 and IL-10, in the presence of media conditioned by premalignant lesion cells vs. HNSCC cells at different time points will further delineate the mechanism by which premalignant lesions modulate the immune response and through which cell types.
In addition to cytokine analyses, the current study used surface markers as an alternate way to determine how mediators released by premalignant lesion cells impact on the activation of T cells. Previous studies in the 4-NQO model have shown that an increased percentage of T cells isolated from the cervical lymph nodes of premalignant lesion-bearing mice express markers of activation, including CD25 and CD69, and markers of exhaustion, including PD-1, compared to T cells isolated from the cervical lymph nodes of HNSCC-bearing mice (38). These studies suggest that the premalignant lesion environment supports a more activated, robust immune response compared to the established tumor environment. Determination of how mediators released directly by premalignant lesion cells impact on the activation status of T cells showed that, in the presence of media conditioned by premalignant lesion cells, an increased percentage of CD4+ T cells expressed CD25 compared to T cells in the presence of media conditioned by HNSCC cells or media alone. One explanation for these data is that immune mediators released by premalignant lesion cells induce increased activation of CD4+ T cells by increasing expression of CD25, i.e. IL-2 receptor expression. However, it is also possible that an increased population of CD25+ T regulatory cells is induced in the premalignant lesion environment compared to the HNSCC environment, although this explanation is not supported by previous studies in the 4-NQO model which have shown that cervical lymph nodes of HNSCC-bearing mice contain an increased percentage and absolute number of Foxp3+ T regulatory cells compared to the cervical lymph nodes of premalignant lesion-bearing and control mice (38).
Further analysis of the activation status of T cells in the premalignant lesion vs. HNSCC environments showed that an increased percentage of CD8+ T cells express CD69 in the presence of media conditioned by premalignant lesion cells compared to media conditioned by HNSCC cells or media alone. Furthermore, a decreased percentage of CD4+ T cells expressed CD69 in the presence of media conditioned by HNSCC compared to media alone. Further analysis of CD69 expression showed that, in the presence of media conditioned by premalignant lesion cells, a striking CD69hi-expressing population was induced in both the CD4+ and CD8+ T cell compartments, suggesting that the premalignant lesion environment stimulates significant early activation of a subset of T cells. While previous studies have suggested that the environment of premalignant oral lesions is more immune stimulatory, the current study offers evidence that factors released directly by premalignant lesion cells promote increased activation of T cells, which has not been previously shown.
The current study is not without limitations. Dissecting how premalignant lesion cells and HNSCC cells themselves contribute to the immune environment, and how this impacts on infiltrating immune cells, is difficult for many reasons, one of which is the complexity of a developing tumor. The current study demonstrates that premalignant lesion cell-conditioned media is immune stimulatory, but the identity of the immune-activating factors, and the mechanism of activation, is yet unknown. The strength of establishing cell cultures from premalignant lesions and HNSCC tumors, is that the cytokine and chemokine environment set up by the cells themselves (vs. surrounding tissue), can be defined, allowing future investigation into the mechanism of how premalignant lesions modulate the immune response. To further hone-in on how mediators released directly from premalignant lesion cells and HNSCC cells play a role in both T cell differentiation and activation, future studies should be focused on isolated subsets of immune cells, including CD4+ T cells and CD8+ T cells, at different time points in culture to better define their phenotype and their function.
Oral cancer is a unique malignancy, in that it can be diagnosed when tissue first becomes dysplastic and presents as leukoplakias or erythroplakias developing on the tongue and floor of mouth. While few previous studies have suggested that the premalignant lesion environment is immune stimulatory, the current study demonstrates that mediators released by premalignant lesion cells themselves, set up a proinflammatory environment and elicit increased cytokine production and activation in T cells, and therefore begin to define a mechanism by which premalignant oral lesions modulate the immune response. Importantly, the current study offers evidence that by diagnosing oral lesions before they develop into established HNSCC and administering targeted immunotherapies at this stage, when immune response is not yet suppressed, treatment may become more effective for patients of premalignant oral lesions.
Acknowledgments
This work was supported by the Clinical Sciences Research and Development Program (I01-CX000851) of the Department of Veterans Affairs and by grants from the National Institutes of Health (RO1-CA128837) (MRIY).
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/, posted April 2016. [Google Scholar]
- 2.Grandis JR, Pietenpol JA, Greenberger JS, Pelroy RA, Mohla S. Head and neck cancer: meeting summary and research opportunities. Cancer Res. 2004;64:8126–8129. doi: 10.1158/0008-5472.CAN-04-2445. [DOI] [PubMed] [Google Scholar]
- 3.Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006;28:462–470. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
- 4.Badoual C, Sandoval F, Pere H, Hans S, Gey A, Merillon N, Van Ryswick C, Quintin-Colonna F, Bruneval P, Brasnu D, Fridman WH, Tartour E. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck. 2010;32:946–958. doi: 10.1002/hed.21346. [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(Suppl 7):vii252–261. doi: 10.1093/annonc/mdq453. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert H, Kagan AR. Recurrence patterns in squamous cell carcinoma of the oral cavity, pharynx, and larynx. J Surg Oncol. 1974;6:357–380. doi: 10.1002/jso.2930060502. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, Whiteside TL. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14:3706–3715. [Google Scholar]
- 9.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 10.Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of 1α,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71:659–665. doi: 10.1016/j.humimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, Nazarian R, Califano J, Borrello I, Serafini P. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008;8:10. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WL, Chang WL, Yang HB, Chang IW, Lee CT, Chang CY, Lin JT, Sheu BS. Quantification of tumor infiltrating Foxp3+ regulatory T cells enables the identification of high-risk patients for developing synchronous cancers over upper aerodigestive tract. Oral Oncol. 2015;51:698–703. doi: 10.1016/j.oraloncology.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL, Hou WJ, Wei Y, Wen YH, Wang YJ, Wen WP. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. 2015;33:354–362. doi: 10.3892/or.2014.3553. [DOI] [PubMed] [Google Scholar]
- 16.Malm IJ, Bruno TC, Fu J, Zeng Q, Taube JM, Westra W, Pardoll D, Drake CG, Kim YJ. Expression profile and in vitro blockade of PD-1 in HPV-negative head and neck squamous cell carcinoma. Head Neck. 2015;37:1088–1095. doi: 10.1002/hed.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punt S, Dronkers EA, Welters MJ, Goedemans R, Koljenovic S, Bloemena E, Snijders PJ, Gorter A, van der Burg SH, de Jong RJ, Jordanova ES. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17 cell frequency. Cancer Immunol Immunother. 2016;65:393–403. doi: 10.1007/s00262-016-1805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewett A, Head C, Cacalano NA. Emerging mechanisms of immunosuppression in oral cancers. J Dent Res. 2006;85:1061–1073. doi: 10.1177/154405910608501201. [DOI] [PubMed] [Google Scholar]
- 19.Abrahao AC, Castilho RM, Squarize CH, Molinolo AA, dos Santos-Pinto D, Jr, Gutkind JS. A role for COX2-derived PGE2 and PGE2-receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol. 2010;46:880–887. doi: 10.1016/j.oraloncology.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker C, Kristensen F, Bettens F, deWeck AL. Lymphokine regulation of activated (G1) lymphocytes. I Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983;130:1770–1773. [PubMed] [Google Scholar]
- 21.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahl SM, Wen J, Moutsopoulos N. TGF-β: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 23.Nitsch SM, Pries R, Wollenberg B. Head and neck cancer triggers increased IL-6 production of CD34+ stem cells from human cord blood. In Vivo. 2007;21:493–498. [PubMed] [Google Scholar]
- 24.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodford D, Johnson SD, De Costa AM, Young MR. An inflammatory cytokine milieu is prominent in premalignant oral lesions, but subsides when lesions progress to squamous cell carcinoma. J Clin Cell Immunol. 2014;5:1–17. doi: 10.4172/2155-9899.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juretic M, Cerovic R, Belusic-Gobic M, Brekalo Prso I, Kqiku L, Spalj S, Pezelj-Ribaric S. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol. 2013;59:99–102. [PubMed] [Google Scholar]
- 27.Rhodus NL, Cheng B, Myers S, Miller L, Ho V, Ondrey F. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. 2005;44:77–82. doi: 10.1002/mc.20113. [DOI] [PubMed] [Google Scholar]
- 28.Bondad-Palmario GG. Histological and immunochemical studies of oral leukoplakia: phenotype and distribution of immunocompetent cells. J Philipp Dent Assoc. 1995;47:3–18. [PubMed] [Google Scholar]
- 29.Ohman J, Magnusson B, Telemo E, Jontell M, Hasseus B. Langerhans cells and T cells sense cell dysplasia in oral leukoplakias and oral squamous cell carcinomas – evidence for immunosurveillance. Scand J Immunol. 2012;76:39–48. doi: 10.1111/j.1365-3083.2012.02701.x. [DOI] [PubMed] [Google Scholar]
- 30.Costa NL, Goncalves AS, Souza-Lima NC, Jaime-Paiva LG, Junqueira-Kipnis AP, Silva TA, Mendonca EF, Batista AC. Distinct expression of perforin and granzyme B in lip and oral cavity squamous cell carcinoma. J Oral Pathol Med. 2011;40:380–384. doi: 10.1111/j.1600-0714.2011.01014.x. [DOI] [PubMed] [Google Scholar]
- 31.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 32.Schoop RA, Noteborn MH, Baatenburg de Jong RJ. A mouse model for oral squamous cell carcinoma. J Mol Histol. 2009;40:177–181. doi: 10.1007/s10735-009-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 34.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins BL, Heniford BW, Ackermann DM, Leonberger M, Martinez SA, Hendler FJ. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 1994;16:424–432. doi: 10.1002/hed.2880160506. [DOI] [PubMed] [Google Scholar]
- 36.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655–667. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Smith LP, Thomas GR. Animal models for the study of squamous cell carcinoma of the upper aerodigestive tract: a historical perspective with review of their utility and limitations. Part A Chemically-induced de novo cancer, syngeneic animal models of HNSCC, animal models of transplanted xenogeneic human tumors. Int J Cancer. 2006;118:2111–2122. doi: 10.1002/ijc.21694. [DOI] [PubMed] [Google Scholar]
- 38.De Costa AM, Schuyler CA, Walker DD, Young MR. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2012;61:927–939. doi: 10.1007/s00262-011-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson SD, De Costa AM, Young MR. Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer. Cancers. 2014;6:756–770. doi: 10.3390/cancers6020756. [DOI] [PMC free article] [PubMed] [Google Scholar]


