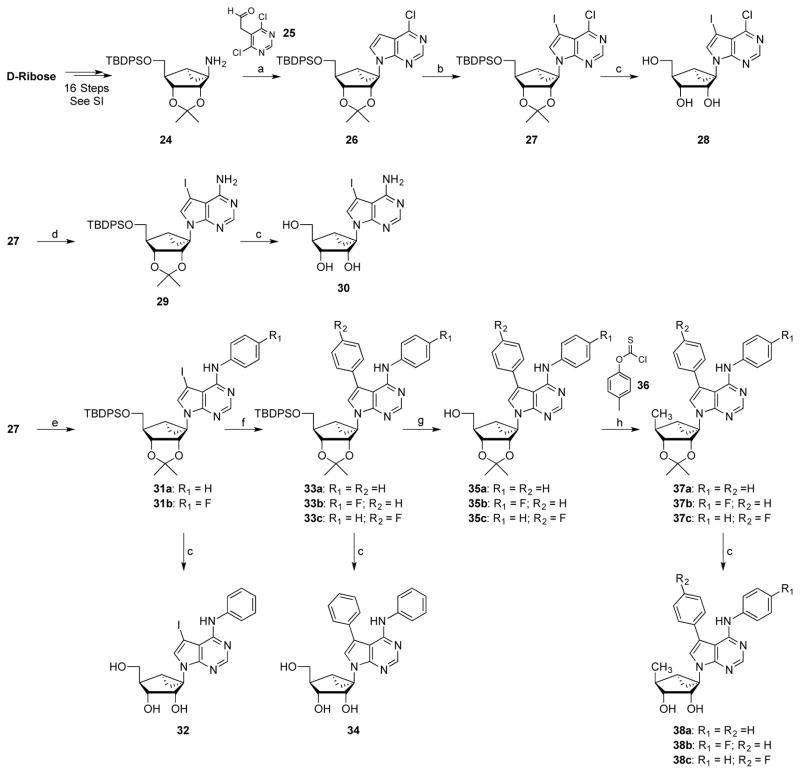
Scheme 1.
Synthesis of nucleobase modified (S)-methanocarba analogues of 5-iodotubercidin. Reagents and conditions: (a) EtOH, 2-(4,6-dichloropyrimidin-5-yl)acetaldehyde (25), NEt3, reflux, 18 h, 76%; (b) anhydrous DMF, NIS, 60 °C, 6 h, 92%; (c) 70% TFA (aq), rt, 1.5 h, from 27–83%; (d) EtOH, 7N NH3-MeOH, sealed tube, 110 °C, 18 h, 89%; (e) anhydrous THF, aniline, 1M tBuOK in THF, −20 °C, 1.5 h, 70–80%; (f) 6:1 DME-EtOH, phenylboronic acid, Pd(PPh3)4, sat. Na2CO3 (aq), 90 °C, 7 h, 75–86%; (g) anhydrous THF, 1M TBAF in THF, rt, 18 h, 85–96%; (h) (i) anhydrous CH3CN, DMAP, O-(p-tolyl) chlorothionoformate (36), rt, 18 h; (ii) anhydrous toluene, Bu3SnH, AIBN, reflux, 2 h, 52–90%.