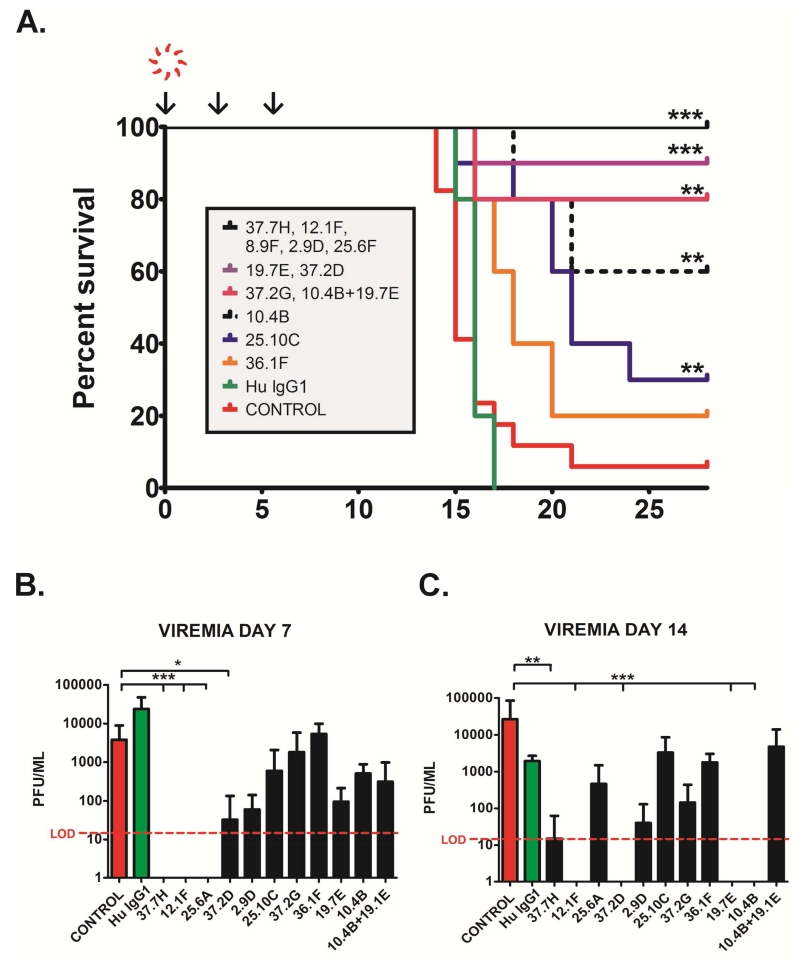
Figure 2. Protective efficacy profile of LASV GPC huMAbs in a Hartley GP model of LF.
(A.) Pooled survival plots for treated and control GPs challenged with LASV from multiple independent experiments, as noted. Control GPs succumbed to LF between 14 and 22 PI (median = 15 days). In these studies a single GP survived infection through the 28-day study endpoint (1/18). Hartley GPs challenged with GPA LASV strain Josiah on day 0 ( ) were treated with LASV huMAbs, at 30 mg/kg of body weight on the same day (↓). Additional therapeutic antibody was administered at the same dose on days 3 and 6 PI (↓). Animals were monitored for clinical signs of LF throughout the 28-day study timeline. (B.) Viremia data from treated and control GP plasma on days 7 and 14 PI. Viremia levels for day 7 treatment groups 37.7H, 12.1F, and 25.6A as well as day 14 12.1F, 37.2D, 19.7E, and 10.4B were below the limit of detection (LOD). Error bars represent standard deviation from mean values. * denotes P≤0.05. ** denotes P≤0.001. *** denotes P≤0.0001.
) were treated with LASV huMAbs, at 30 mg/kg of body weight on the same day (↓). Additional therapeutic antibody was administered at the same dose on days 3 and 6 PI (↓). Animals were monitored for clinical signs of LF throughout the 28-day study timeline. (B.) Viremia data from treated and control GP plasma on days 7 and 14 PI. Viremia levels for day 7 treatment groups 37.7H, 12.1F, and 25.6A as well as day 14 12.1F, 37.2D, 19.7E, and 10.4B were below the limit of detection (LOD). Error bars represent standard deviation from mean values. * denotes P≤0.05. ** denotes P≤0.001. *** denotes P≤0.0001.