Abstract
Background:
Dyslipidemia is an important risk factor for cardiovascular disease but is suboptimally managed. Pharmacists are accessible primary care professionals and with expanded scopes of practice (including prescribing), could identify and manage patients with dyslipidemia. We sought to evaluate the effect of pharmacist prescribing of dyslipidemia medications on the proportion of participants achieving target LDL-cholesterol (LDL-c) levels.
Methods:
We conducted a randomized controlled trial in 14 community pharmacies in Alberta, Canada. We enrolled adults with uncontrolled dyslipidemia as defined by the 2009 Canadian Dyslipidemia Guidelines. Intervention was pharmacist-directed dyslipidemia care, including assessment of cardiovascular risk, review of LDL-c, prescribing of medications, health behaviour interventions and follow-up every 6 weeks for 6 months. Usual care patients received their lipid results and a pamphlet on cardiovascular disease and usual care from their physician and pharmacist. Primary outcome was the proportion of participants achieving their target LDL-c (<2 mmol/L or ≥50% reduction) at 6 months between groups.
Results:
We enrolled 99 patients with a mean (SD) age of 63 (13) years, 49% male and baseline LDL-c of 3.37 mmol/L (0.98). Proportion of patients achieving LDL-c target was 43% intervention versus 18% control (p = 0.007). Adjusted odds of achieving target LDL-c were 3.3 times higher for the intervention group (p = 0.031), who also achieved greater reduction in LDL-c (1.12 mmol/L, SE = 0.112) versus control (0.42 mmol/L, SE = 0.109), for an adjusted mean difference of 0.546 mmol/L (SE = 0.157), p < 0.001.
Conclusion:
Pharmacist prescribing resulted in >3-fold more patients achieving target LDL-c levels. This could have major public health implications.
Knowledge Into Practice.
Using a systematic, case-finding approach, pharmacists can efficiently identify patients with untreated or inadequately treated dyslipidemia risk.
In a randomized trial of pharmacist prescribing in patients with dyslipidemia, greater than 3-fold more patients reached their LDL cholesterol goal, compared to usual care.
This is the first randomized trial of independent pharmacist prescribing and an advanced scope of practice in patients with dyslipidemia.
Mise en pratique des connaissances.
À l’aide d’une méthode de recherche de cas systématique, les pharmaciens peuvent repérer efficacement les patients qui présentent un risque de dyslipidémie non traité ou traité de façon inadéquate.
Dans le cadre d’une étude aléatoire portant sur des pharmaciens délivrant des ordonnances à des patients atteints de dyslipidémie, un nombre plus de trois fois supérieur de patients ont atteint leur objectif de LDL-cholestérol par rapport aux soins habituels.
Il s’agit de la première étude aléatoire sur des pharmaciens indépendants délivrant des ordonnances et d’un élargissement du champ d’activité aux patients atteints de dyslipidémie.
Introduction
Heart disease is the number one cause of death for men and women in the United States,1 and cardiovascular disease (CVD) is the cause of one-third of all deaths in Canada.2 One important risk factor for CVD is dyslipidemia. The National Health and Nutrition Examination Survey from 2003-2006 found that roughly 32% of Americans aged 50 to 64 had unhealthy levels of LDL cholesterol (LDL-c).3 The Canadian Health Measures survey, conducted from 2007 to 2009, found that roughly 36% of all Canadians had unhealthy levels of LDL cholesterol (LDL-c), and this prevalence increased with age, peaking at 43% in those aged 40 to 59.4 Despite strong evidence and clear practice guidelines for the management of this risk factor, it remains suboptimally treated.5-10 Some of this represents undertreatment due to patients not presenting to or failing to be screened by primary care physicians, a reluctance to initiate or titrate lipid-lowering therapies,6 or because of poor adherence or reluctance to initiate medications by patients.8,11
Pharmacists are front-line primary care professionals who see many patients at risk for CVD, often more frequently than physicians.12 A number of recent systematic reviews have demonstrated the effectiveness of a variety of pharmacists’ interventions, including in the management of patients with dyslipidemia.13,14 Furthermore, in 2007, pharmacists in the province of Alberta, Canada, were granted the ability to apply for independent prescribing authority, allowing them to prescribe medications.15 To qualify to apply for this authority, pharmacists must have been in practice for at least 1 year and demonstrate a high level of clinical care and management through the submission of a detailed application and 3 patient cases.16 In addition, all Alberta pharmacists have also been extended the authority to order and interpret laboratory tests for patients.17
Pharmacists are thus well positioned to systematically and proactively identify patients with unrecognized or undertreated dyslipidemia as a public health approach to chronic disease management. To our knowledge, there have not been any studies examining the outcomes associated with pharmacists’ use of independent prescribing authority to manage patients with dyslipidemia.
The primary objective of this study was to evaluate the effect of pharmacist intervention (participant identification, assessment, care plan development, education/counselling, prescribing/titration of lipid-lowering medications and close follow-up) on the proportion of participants achieving target LDL-c levels as defined by the 2009 Canadian Cardiovascular Society (CCS) dyslipidemia guidelines.2 Secondary objectives included determining the effects of the intervention on the difference in change in LDL-c and apolipoprotein-B (Apo-B) between groups at 6 months.
Methods
Design
This was a randomized controlled trial of pharmacist intervention, with the unit of randomization being the patient. Due to the nature of the intervention, blinding was not possible in this study.
Setting
Fourteen community pharmacies from across Alberta, Canada, participated in patient recruitment and follow-up. Each of the 14 sites had at least 1 pharmacist with additional prescribing authorization. Pharmacist investigators were recruited through our pharmacy practice research network.
Patient population
The participant population was comprised of adults with uncontrolled dyslipidemia as defined by the 2009 Canadian Dyslipidemia Guidelines.2 Participants were not required to be treatment naïve but had to be willing to take statin therapy if prescribed by the pharmacist.
Participant identification/screening and enrollment
Pharmacists were encouraged to apply the principles of “case finding” as part of the recruitment procedures for this study.21 Case finding is the process by which health care professionals use patient characteristics such as demographics, risk factors and symptoms to direct their decision-making around whether or not that patient should undergo further testing for a particular condition.21 All risk factors and symptoms used in the identification of possibly eligible patients for this study were gathered from the guidelines developed for the treatment of dyslipidemia.2,22
Eligibility criteria: All eligibility criteria were based on the 2009 CCS dyslipidemia guidelines.2 To be included in the study, all participants had to be >18 years of age and have suboptimally controlled dyslipidemia defined by their CVD risk, as follows: (1) high-risk patients, including those with known coronary artery disease (CAD), cerebrovascular disease (stroke/transient ischemic attack), peripheral arterial disease, diabetes, or a calculated Framingham Risk Score (FRS) of ≥20% and LDL-c ≥2 mmol/L; (2) moderate-risk patients included patients with a calculated FRS of 10% to 19% and LDL-c >3.5 mmol/L if not treated or LDL-c ≥2 mmol/L if treated; or (3) moderate risk (FRS 10%-19%) in treatment-naïve males >50 years or females >60 years with an LDL-c of ≤3.5 mmol/L and hs-CRP ≥2 mg/L (measured twice 1 to 2 weeks apart); or (4) low risk (FRS <10% and LDL-c ≥5.0 mmol/L).2,23 We excluded patients who were unwilling/unable to use statins, were pregnant or nursing, or had renal impairment (defined as a creatinine clearance ≤30 mL/min using the Modification of Diet in Renal Disease [MDRD] study equation) or significant hepatic dysfunction (alanine aminotransferase [ALT] levels >120 U/L).
Randomization
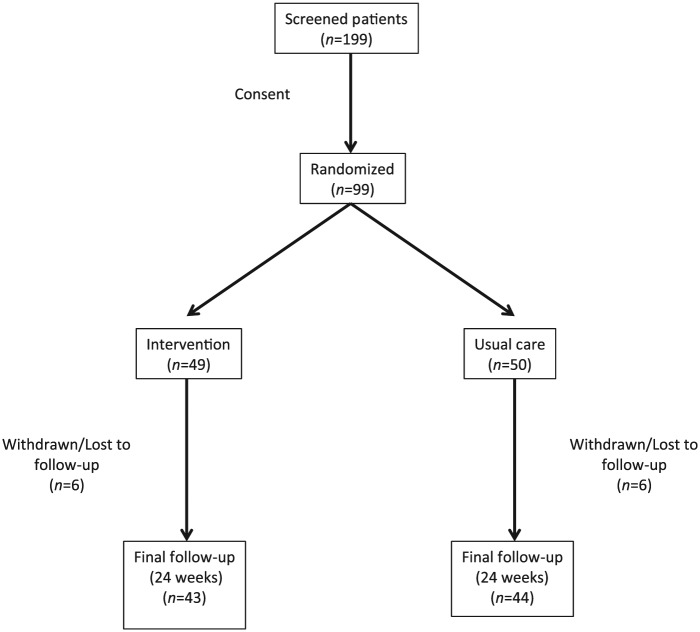
Once eligibility had been determined and the participant provided written informed consent, the participant was randomized in a 1:1 ratio via a secure website, managed by the Epidemiology Coordinating and Research Centre (EPICORE, www.epicore.ualberta.ca), to ensure allocation concealment to either pharmacist intervention or usual care (Figure 1). Randomization was stratified by study centre (pharmacy), and a variable block size was used.
Figure 1.
Treatment algorithm
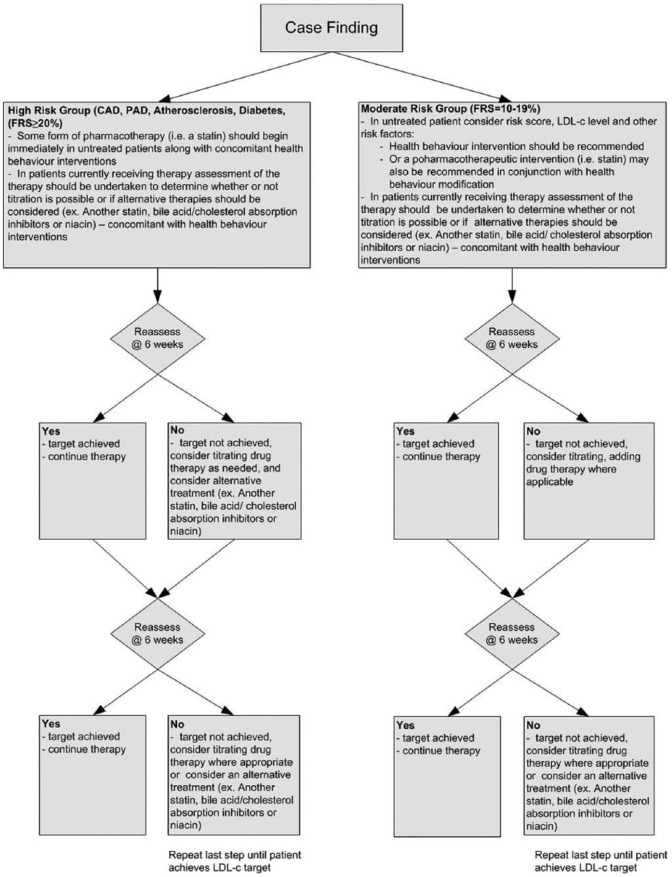
Intervention group: The prescribing intervention was pharmacist-directed dyslipidemia care based on the 2009 CCS dyslipidemia guidelines.2 Pharmacists assessed each participant’s overall cardiovascular risk, including reviewing LDL-c control, developed treatment goals and determined health behaviour interventions (e.g., smoking cessation, diet and exercise) for the participant to undertake to help them manage their overall CV risk. Pharmacists worked with the participant to determine the best treatment option and approach, along with a plan for the implementation of these strategies. In brief, the treatment algorithm can be found in Figure 2.
Figure 2.
Study flow diagram
In the intervention group, pharmacists prescribed dyslipidemia therapies as indicated. Whenever drug therapy was initiated or a dosage adjusted, the prescribing pharmacist ordered all appropriate laboratory tests (e.g., fasting lipid panel, apo-B, creatine kinase [CK], ALT, creatinine, fasting blood sugars, A1c for diabetic patients) and monitored the participant to ensure the treatment’s efficacy and safety. Additionally, at each visit, participants were assessed for drug tolerability, including symptoms of myalgia and gastrointestinal tolerance. If any adverse event was noted, pharmacists intervened as necessary to ensure participant safety. Pharmacists worked closely with participants to determine the optimal drug therapy choices for each individual participant, taking into account drug interactions, medical conditions and degree of LDL-c lowering required.
Participants in the intervention group received a copy of their laboratory results, their calculated FRS (if applicable) and a participant information package on dyslipidemia.24 Physicians involved in the care of study participants randomized to the intervention group in the study were made aware of participants’ involvement in the study. The physician was also informed of all changes made to the participant’s drug therapy regimen, as per the requirements of the Alberta College of Pharmacists.25
Participants received follow-up in person or over the telephone, at 6, 12, 18 and 24 weeks post-randomization. Participants were given a laboratory requisition to have follow-up fasting lipid panel, along with treatment-monitoring lab tests (e.g., ALT, CK), performed as needed and prior to each follow-up visit, to monitor treatment safety and efficacy. Interim telephone follow-up may have been performed at the discretion of the pharmacist investigator. Other cardiovascular risk reduction interventions, including discussion of smoking cessation, were performed at the discretion of the pharmacist.
Control Group (Usual Care)
Participants randomized to the control group received a copy of their lab results, a pamphlet on cardiovascular disease26 and usual care from their pharmacist and physician. Usual care participants were seen at 12 and 24 weeks post-randomization. The rationale for including the 12-week visit was to minimize loss to follow-up. Participants in this group were also given a laboratory requisition to have follow-up fasting lipid profiles, Apo-B, CK and ALT measures performed prior to each visit. Physicians of a participant who had been randomized to the usual care group were not actively informed of their participation in the study, but participants were encouraged to discuss their LDL-c levels with their physicians.
Outcomes: The primary outcome of this study was the proportion of participants achieving their target LDL-c (<2 mmol/L or ≥50% reduction in LDL-c) at 6 months in the intervention versus usual care groups. The secondary outcomes included the difference in change in LDL-c between the intervention and usual care groups at 6 months and the difference in change in Apo-B between baseline and 6 months in intervention patients.
Sample size: Based on a review of recent literature,18-20 we hypothesized that 70% of intervention participants would reach target LDL-c levels (<2 mmol/L for high- and moderate-risk patients and a 50% reduction in LDL-c, from baseline, for low-risk patients) at 6 months. We estimated a sample size of 82 would provide 80% power to detect a difference of 30% between the groups (based on a 2-sided α of 0.05). As a pragmatic, practice-based trial, the sample size was increased to 100 to account for possible losses due to follow-up and withdrawals.
Statistical analyses: All analyses were based on intention to treat principles and using a last value carried forward approach to missing data. A comparison of baseline characteristics was performed using t tests or nonparametric Wilcoxon test for continuous variables and chi-square test for categorical variables. The primary outcome, proportion of participants to reach target LDL-c levels, was analyzed by the chi-square test and binary logistic regression to adjust for any baseline differences between variables (p < 0.1). We adjusted for the clustering effect by performing generalized estimating equations with binomial family. Since the number of clusters was small (only 14), we used the MBN small-sample correction method.27 The secondary outcomes were analyzed using ANCOVA, adjusting for differences in baseline variables that were different at p < 0.1.
Ethical considerations
The Health Research Ethics Board at the University of Alberta approved the study protocol and procedures, and all patients provided written informed consent to participate.
Results
Between December 2011 and July 2013, 199 possibly eligible patients were screened (Figure 2); of these, 99 patients were eligible for participation in the study and randomized (49 patients were randomized to intervention and 50 patients were randomized to usual care). Of those 100 patients who were not eligible for participation, the majority did not have elevated levels of LDL-c as defined by the dyslipidemia guidelines2; other patients did not complete baseline testing of their LDL-c levels, were not interested in participating in the study or could not tolerate taking a statin if one were prescribed. A total of 12 patients withdrew from the study early (6 in each group).
Intervention and usual care groups were similar at baseline, as described in Table 1, with the exception of the frequency of a diagnosis of heart failure between the groups. The average age of participants was 63 years (SD = 12.6), and there was an equal distribution of men and women enrolled. Most of the primary prevention participants had a high level of risk according to the Framingham Risk Score. About one-third of patients were taking a statin at baseline.
Table 1.
Study population
| Characteristic | Usual care group (n = 50) Mean (SD)/prevalence, (n) | Intervention group (n = 49) Mean (SD)/prevalence (n) | |
|---|---|---|---|
| Mean age | 63 (11.91) | 63 (13.34) | |
| Gender | Male | 52% (26) | 47% (23) |
| Mean baseline LDL-c (mmol/L) | 3.21 (0.81) | 3.52 (1.12) | |
| Mean systolic blood pressure (mmHg) | 133 (18.39) | 129 (14.14) | |
| Mean diastolic blood pressure (mmHg) | 79 (10.47) | 77 (10.05) | |
| Mean BMI | 31.35 (5.55) | 31.80 (10.49) | |
| Baseline Framingham Risk Score (primary prevention patients) | High risk | 32% (23) | 27% (19) |
| Moderate risk | 13% (9) | 17% (12) | |
| Low risk | 4% (3) | 7% (5) | |
| Secondary prevention patients | 27% (14) | 27% (13) | |
| Baseline statin prescription | 42% (21) | 31% (15) | |
| Cerebrovascular disease/stroke/TIA | 12% (6) | 8% (4) | |
| Coronary heart disease/acute MI | 10% (5) | 16% (8) | |
| Diabetes | 43% (21) | 57% (28) | |
| Heart failure | 0%* | 8%* (4) | |
| Lower extremity PAD | 6% (3) | 2% (1) | |
MI, myocardial infarction; PAD, peripheral arterial disease; TIA, transient ischemic attack.
Denotes significant differences noted between the usual care and intervention groups.
Primary outcome
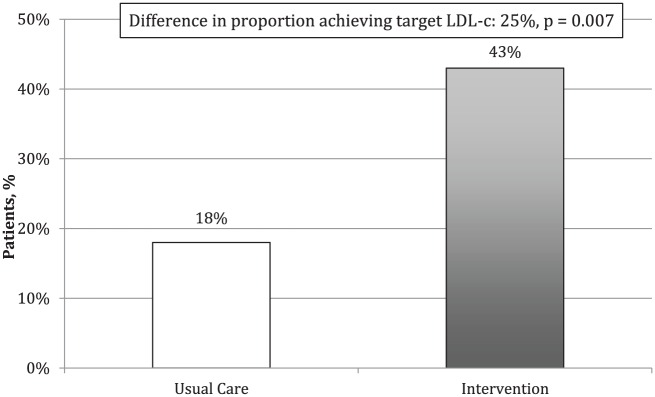
The proportion of patients achieving the 2009 CCS dyslipidemia guidelines target for LDL-c levels at 6 months was 43% for the intervention group and 18% for usual care group, for an absolute difference of 25% (p = 0.007) (Figure 3). The odds (adjusted for cluster effect) of reaching target LDL-c was 3.30 (95% CI, 1.12-9.78, p = 0.031).
Figure 3.
Proportion of patients achieving target LDL-c levels after 6 months
Secondary outcomes
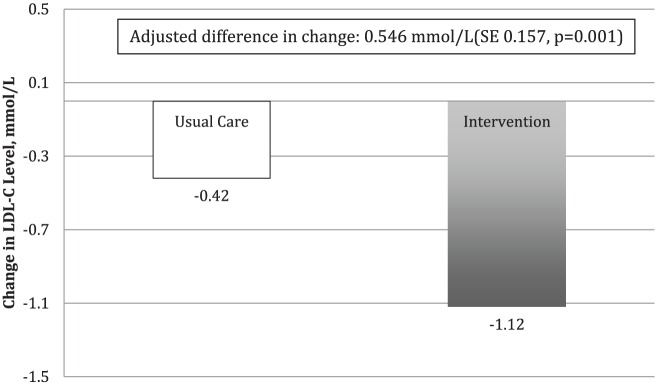
A reduction in LDL-c levels was observed for both the intervention and usual care groups within this study. However, a reduction in LDL-c levels for the intervention group of 1.12 mmol/L (SE = 0.112) was significantly greater than the reduction of 0.42 mmol/L (SE = 1.09) achieved by the usual care group. The adjusted difference in change between intervention and usual care groups was 0.546 mmol/L (SE = 0.157, p = 0.001) (Figure 4). There was no significant difference in the change in apo-B between the groups from baseline to 6 months. However, these data were not consistently collected as part of regular laboratory testing during the study, as it is only considered an alternate lipid target in the CCS guidelines. At 6 months, 74% of patients in the intervention group were taking a statin. There were no adverse events reported in either group.
Figure 4.
Adjusted mean change in LDL-c level over 6 months
Discussion
To our knowledge, this is the first randomized trial of independent pharmacist prescribing in the care of patients with dyslipidemia. We found that pharmacist intervention, which included prescribing of lipid-lowering therapies, resulted in an absolute 25% more patients achieving guideline targets for LDL-c levels, a >3-fold improvement compared to usual care. Patients in the intervention group also experienced a greater LDL-c reduction than those patients in the usual care group. Applied on a broader scale, this may help counteract “clinical inertia” and help more patients to achieve lipid targets, which is a major public health problem.
In a recently published systematic review of 21 randomized trials examining pharmacist care for patients with dyslipidemia, authors found that patients receiving pharmacist care had a 0.28 mmol/L greater decrease in their LDL-c levels when compared to control.14 This review also found that patients in the pharmacist intervention groups were 2.46 (95% CI, 1.43-4.25) times more likely to achieve recommended guideline targets for LDL-c levels.14 Most of these studies were limited to pharmacists making recommendations or patient education on adherence. As suggested in the analysis of the SCRIP-HTN study, there may be a ceiling effect for the efficacy of pharmacist interventions when pharmacists only offer suggestions for changes to patients’ medication therapy, as they depend on the individual patient’s physician accepting and implementing the recommendation.28 The pharmacists in our study were able to use their clinical judgement and actively make adjustments to patients’ medication therapy as needed by assuming responsibility for prescribing.
Extrapolating our results to those of the Cholesterol Treatment Trialists’ Collaboration meta-analysis of data from 90,056 participants from 14 randomized trials of statins, a number of important clinical implications can be identified.29 From these figures, we estimate that a sustained 0.546 mmol/L LDL-c difference between the intervention and usual care groups (as found in our trial) would translate into a 13% reduction in coronary death or nonfatal MI, 13% reduction in coronary revascularization and 11% reduction in ischemic stroke. Given the high prevalence of dyslipidemia in Western civilization, these findings have public health importance.
Limitations
There are a number of limitations in this study that warrant comment. To begin, due to the nature of the study, it was not possible to blind patients or pharmacists to the treatment group to which they were allocated (although our outcome measure was objective). Second, it was not possible to completely measure change in apo-B, one of our secondary outcomes, as laboratory results were not consistently collected (they were often not available from the local laboratory). Third, as only about 25% of Alberta pharmacists currently have additional prescribing authority (although this number is growing rapidly), it is possible that the group of pharmacists participating in this study is not representative of the wider population of practising pharmacists. Conversely, the level of care provided by this select group of pharmacists to usual care patients cannot be assumed to be equal to the “usual care” in other pharmacy settings. In fact, our usual care arm (with a reduction of LDL-c of 0.42 mmol/L) may be superior to “usual care” in the community and may have introduced a bias toward the null hypothesis. This was a trial of a treatment approach—having pharmacists assess and prescribe based on the CCS dyslipidemia guidelines—and as such, we were not able to obtain fine detail about specific actions such as patient education, prescribing, dosage adjustment, adherence assessment and so on, which might provide “mechanistic insight.” Finally, while the sample size might appear “small,” we assumed a large effect size, which was achieved by our intervention.
The results of this study demonstrate that independent pharmacist prescribing yields a clinically significant increase in the proportion of patients achieving guideline treatment targets in the management of dyslipidemia and a reduction in LDL-c levels. This study, which is the first to examine the efficacy of independent prescribing by pharmacists in patients with dyslipidemia, lends further support to the value of pharmacist-directed medication therapy management.
Acknowledgments
RxACT Investigators: Rita Bowron, Safeway Pharmacy, Calgary, Alberta; Lonni Johnson, Winter’s Pharmacy, Drayton Valley, Alberta; Rita Lyster, Rita’s Apothecary & Home Healthcare, Barrhead, Alberta; Anita Brown and Sandra Trkulja, Shoppers Drug Mart, Okotoks, Alberta; Carol Wei, Safeway Pharmacy, Airdrie, Alberta; Sheilah Kostecki, Safeway Pharmacy, Calgary, Alberta; Darsey Milford, Turtle Mountain Pharmacy, Bellevue, Alberta; Candice Edgecombe, Safeway Pharmacy, Edmonton, Alberta; Teryn Wasileyko, Safeway Pharmacy, Edmonton, Alberta; Tony Nickonchuk, formerly of Wal-Mart Pharmacy, Peace River, Alberta; Louise Sharren, Safeway Pharmacy, St. Albert, Alberta; Rick Siemens, London Drugs, Lethbridge, Alberta; Anita Dobson, Calgary, Alberta; and Nader Hammoud, formerly of Safeway Pharmacy, Calgary, Alberta.
Footnotes
Author Contributions:R. Tsuyuki wrote the original protocol, obtained funding and oversaw the project. M. Rosenthal was the project manager and conducted the analyses. G. Pearson provided the education for the pharmacists and provided input into the study protocol. All participated in the writing of the manuscript.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:RxACT was supported by an investigator-initiated grant from AstraZeneca. The sponsor had no role in the protocol design, study conduct, analysis/interpretation of the findings or decision to publish.
References
- 1. Heart disease: scope and impact USA2014. www.theheartfoundation.org/heart-disease-facts/heart-disease-statistics/ (accessed April 23, 2014).
- 2. Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can J Cardiol 2009;25:567-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toth PP, Potter D, Ming EE. Prevalence of lipid abnormalities in the United States: the National Health and Nutrition Examination Survey 2003–2006. J Clin Lipidology 2012;6:325-30. [DOI] [PubMed] [Google Scholar]
- 4. Canadian health measures survey: cholesterol and vitamin D levels 2010. www.statcan.gc.ca/daily-quotidien/100323/dq100323a-eng.htm (accessed Feb. 24, 2014).
- 5. Petrella R, Merikle E. A retrospective analysis of the prevalence and treatment of hypertension and dyslipidemia in Southwestern Ontario, Canada. Clinical Therapeutics 2007;39(4):742-50. [DOI] [PubMed] [Google Scholar]
- 6. Goodman S, Bastien N, McPherson R, Francis G, Genest JJ, Leiter L. Prevalence of dyslipidemia in statin-treated patients in Canada: results of the DYSlipidemia International Study (DYSIS). Can J Cardiol 2010;26(9):e330-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonald M, Hertz R, Unger A, Lustik M. Prevalence, awareness and management of hypertension, dyslipidemia and diabetes among United States adults aged 65 and older. J Gerontol A Biol Sci Med Sci 2009;64A(2):256-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CQIN Investigators. Low incidence of assessment and modification of risk factors in acute care patients at high risk for cardiovascular events, particularly among females and the elderly. Am J Cardiol 1995;76:570-73. [DOI] [PubMed] [Google Scholar]
- 9. Tsuyuki R, Bungard T. Poor adherence with hypolipidemic medications: a lost opportunity. Pharmacotherapy 2001;21:576-82. [DOI] [PubMed] [Google Scholar]
- 10. Olson K, Bungard T, Tsuyuki RT. Cholesterol risk management: a systematic examination of the gap from evidence to practice. Pharmacotherapy 2001;21:807-17. [DOI] [PubMed] [Google Scholar]
- 11. Grover S, Lowensteyn I, Joseph L, et al. Patient knowledge of coronary risk profile improves the effectiveness of dyslipidemia therapy: the CHECK-IP study: a randomised controlled trial. Arch Intern Med 2007;167(21):2296-303. [DOI] [PubMed] [Google Scholar]
- 12. Tsuyuki R, Johnson J, Teo K, et al. A randomised trial of the effect of community pharmacist intervention on cholesterol risk management: the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP). Arch Intern Med 2002;162:1149-55. [DOI] [PubMed] [Google Scholar]
- 13. Machado M, Bajcar J, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part III: systematic review and meta-analysis in hyperlipidemia management. Ann Pharmacotherapy 2008;42(9):1195-207. [DOI] [PubMed] [Google Scholar]
- 14. Charrois TL, Zolezzi M, Koshman SL, et al. A systematic review of the evidence for pharmacist care of patients with dsylipidemia. Pharmacotherapy 2012;32(3):222-33. [DOI] [PubMed] [Google Scholar]
- 15. Yuksel N, Eberhart G, Bungard T. Prescribing by pharmacists in Alberta. Am J Health-Sys Pharm 2008;65:2126-32. [DOI] [PubMed] [Google Scholar]
- 16. Prescribing authorization application. https://pharmacists.ab.ca/nRegistrationLicensure/Pharmacistsid=6253.aspx (accessed Feb. 27, 2014).
- 17. Summary of pharmacists’ expanded scope of practice activities across Canada. http://blueprintforpharmacy.ca/docs/resource-items/pharmacists’-expanded-scope-of-practice_summary-chart—cpha—january-2014-from-graphicsDF4DC970F6835A01BE1C1989.pdf (accessed Feb. 27, 2014).
- 18. Goff D, Bertoni A, Kramer H, et al. Dyslipidemia prevalence, treatment and control in the multi-ethnic study of athersclerosis (MESA): gender, ethnicity and coronary artery calcium. Circulation 2006;113:647-56. [DOI] [PubMed] [Google Scholar]
- 19. Mohiuddin S, Thakker K, Setze C, Kell M. Evaluating optimal lipid levels in patients with mixed dyslipidemia following short- and long-term treatment with fenofibric acid and statin combination therapy: a post hoc analysis. Curr Med Res Opinion 2011;27(5):1067-78. [DOI] [PubMed] [Google Scholar]
- 20. Weaver J, McManus J, Leung T, et al. Impact of pharmacy-led dyslipidemia interventions on medication safety and therapeutic failure in patients. Rockville, MD: Agency for Healthcare Research and Quality (US); 2005. [PubMed] [Google Scholar]
- 21. Kassamali A, Houle S, Rosenthal M, Tsuyuki RT. Case finding: the missing link in chronic disease management. Can Pharm J (Ott) 2011;144(4):170-71. [Google Scholar]
- 22. Pearson G, Thompson A, Semchuk W. Guidelines for the management of dyslipidemia and prevention of cardiovascular disease by pharmacists. Can Pharm J (Ott) 2008;141(suppl 2):S11-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ridker PM. The JUPITER trial; results, controversies and implications for prevention. Circ Cardiovasc Qual Outcomes 2009;2:279-85. [DOI] [PubMed] [Google Scholar]
- 24. Heart and Stroke Foundation. Living with cholesterol: cholesterol and health living 2013. www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3751077/k.6942/Living_with_Cholesterol.htm (accessed March 13, 2014).
- 25. Alberta College of Pharmacists. Standards of practice 2012. https://pharmacists.ab.ca/npharmacistResources/StandardsofPractice.aspx (accessed March 13, 2014).
- 26. Government of British Columbia. Preventing cardiovascular disease 2008. www.bcguidelines.ca/pdf/cvd_patient_guide.pdf (accessed March 13, 2014).
- 27. Morel JG, Bokossa MC, Neerchal MK. Small sample correction for the variance of GEE estimators. Biometrical J 2003;45(4):395-409. [Google Scholar]
- 28. McLean D, McAlister F, Johnson J, et al. A randomised trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: Study of Cardiovascular Risk Intervention by Pharmacists–Hypertension (SCRIP-HTN). Arch Intern Med 2008;168:2355-61. [DOI] [PubMed] [Google Scholar]
- 29. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. [DOI] [PubMed] [Google Scholar]