Figure 1.
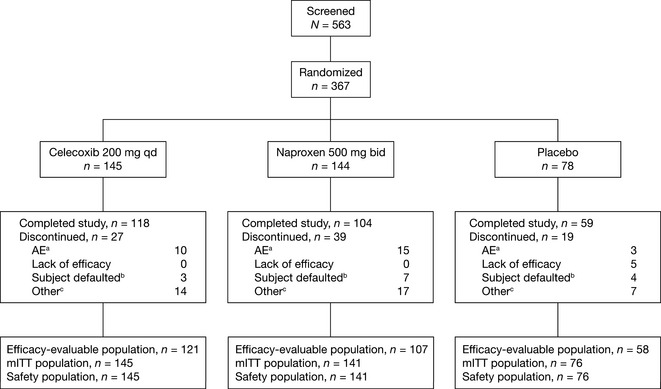
Patient disposition. AE, adverse event; bid, twice daily; mITT, modified intent‐to‐treat; qd, once daily. aIncludes both treatment‐related and non‐treatment‐related AEs. bIncludes ‘lost to follow‐up’ and ‘subject no longer willing to participate in study’. cIncludes ‘protocol violation’.
