Abstract
Combination of tumor antigens with immunostimulants is a promising approach in cancer immunotherapy. We assessed animal model toxicity of AS15 combined with various tumor antigens: WT1 (rabbits), or p501, dHER2 and recPRAME (cynomolgus monkeys), administered in seven or 20 dose regimens versus a saline control. Clinical and ophthalmological examinations, followed by extensive post‐mortem pathological examinations, were performed on all animals. Blood hematology and biochemistry parameters were also assessed. Antigen‐specific antibody titers were determined by enzyme‐linked immunosorbent assay. Additional assessments in monkeys included electrocardiography and immunohistochemical evaluations of the p501 expression pattern. Transient increases in body temperature were observed 4 h or 24 h after injections of recPRAME + AS15 and dHER2 + AS15. Edema and erythema were observed up to 1 week after most injections of recPRAME + AS15 and all injections of dHER2 + AS15. No treatment‐related effects were observed for electrocardiography parameters. Mean fibrinogen levels were significantly higher in all treated groups compared to controls, but no differences could be observed at the end of the treatment‐free period. Transient but significant differences in biochemistry parameters were observed post‐injection: lower albumin/globulin ratios (p501 + AS15), and higher bilirubin, urea and creatinine (dHER2 + AS15). Pathology examinations revealed significant increases in axillary lymph node mean weights (recPRAME + AS15) compared to controls. A 100% seroconversion rate was observed in all treated groups, but not in controls. p501 protein expression was observed in prostates of all monkeys from studies assessing p501 + AS15. These results suggest a favorable safety profile of the AS15‐containing candidate vaccines, supporting the use of AS15 for clinical development of potential anticancer vaccines. Copyright © 2015 The Authors. Journal of Applied Toxicology Published by John Wiley & Sons Ltd.
Keywords: immunostimulant, AS15, WT1, PRAME, p501, HER2, antigen‐specific cancer immunotherapy
Short abstract
The aim of the current paper was to assess the safety profile of vaccine candidates containing the AS15 immunostimulant combined with different antigens in two animal models. Several antigens were tested for this purpose: WT1 (rabbits), p501, dHER2 and recPRAME (cynomolgus monkeys). Only transient differences in hematology and biochemical parameters could be observed, while pathology testing revealed no safety concerns. Our findings support the use of AS15 for clinical development of potential immunotherapeutic cancer vaccines.
Introduction
Although a multitude of anticancer therapeutic approaches are available today, standard treatment (chemotherapy, radiotherapy and surgery) does not always prove effective. The effects of conventional treatment are sometimes seen immediately, tumor size may be reduced, but cancer relapse and severe side effects are a substantial risk (Aldrich et al., 2010; Bilusic and Gulley, 2012). Antigen‐specific cancer immunotherapy offers a promising approach by mobilizing the patient's immune system to target tumor cells specifically, thus decreasing the possibility of side effects due to damage of healthy cells (Dimberu and Leonhardt, 2011; Weir et al., 2011).
Tumor antigens alone often do not elicit a strong immune response and must be combined with a suitable immunostimulant (Cluff, 2010). GSK proprietary AS15 immunostimulant is composed of 3‐O‐desacyl‐4′‐monophosphoryl lipid A (monophosphoryl lipid A; MPL), QS‐21 (extract from the soap bark tree [Quillaja saponaria]) and a synthetic oligodeoxynucleotide containing unmethylated CG dinucleotides (CpG ODNs 7909), in a liposomal formulation. Combination of the MAGE‐A3 tumor antigen with AS15 induced cellular and humoral responses that were more robust and a higher clinical activity compared to MAGE‐A3 combined with the AS02B immunostimulant in a phase II clinical trial in patients with melanoma (Kruit et al., 2013). Therefore, AS15 was chosen for further clinical development in phase III trials for the treatment of non‐small cell lung cancer (NSCLC) (NCT00480025) and melanoma (NCT00796445).
The Wilms' tumor gene (WT1) was originally identified as the gene responsible for the childhood kidney cancer, Wilms' tumor (Call et al., 1990; Gessler et al., 1990; Lee and Haber, 2001). WT1 is highly expressed in a number of hematopoietic (such as acute myelocytic, acute lymphocytic, chronic myelocytic leukemia) and solid (including NSCLC and breast cancer) cancers (Nasomyon et al., 2014; Oka et al., 2002; Xu et al., 2013). A breast cancer candidate vaccine combining the WT1 recombinant antigen with the AS15 immunostimulant (WT1 + AS15) is currently in phase I/II clinical development in patients with WT1‐positive breast cancer (NCT01220128).
The p501 antigen (known also as prostein) is uniquely and widely expressed in both normal and cancerous prostate tissues, and can serve as a marker for metastases of prostatic origin (Sheridan et al., 2007). It was also detected in adenocarcinomas of the bladder (Lane et al., 2008). Another vaccine candidate, containing the p501 recombinant antigen combined with the AS15 immunostimulant (p501 + AS15), was evaluated in a phase I/II clinical trial in patients with hormone‐sensitive prostate cancer and rising prostate‐specific antigen (PSA) (NCT00148928).
The human epidermal growth factor receptor 2 (HER2) is overexpressed in 20–30% of breast cancers (Slamon et al., 1987). A candidate cancer vaccine consisting of a truncated recombinant HER2 protein (dHER2) combined with the AS15 immunostimulant (dHER2 + AS15) has been evaluated in a phase I trial in patients with high‐risk breast cancer overexpressing HER2 (NCT00058526), in a phase I/II clinical trial in patients with metastatic breast cancer overexpressing HER2 (NCT00140738), and in a phase I/II clinical trial in patients with metastatic breast cancer overexpressing HER2 and resistant to trastuzumab (NCT00952692) (Hamilton et al., 2012).
The PReferentially expressed Antigen of MElanoma (PRAME) was isolated from several types of cancer and may be directly involved in oncogenesis (Epping and Bernards, 2006; Epping et al., 2005). The recombinant PRAME antigen combined with AS15 (recPRAME + AS15) is currently in clinical development for the treatment of NSCLC (NCT01159964; phase I) and melanoma (NCT01149343; phase I/II). In these studies, recPRAME + AS15 was immunogenic and had a clinically acceptable safety profile (Gutzmer et al., 2012; Pujol et al., 2012).
The non‐clinical safety studies presented here assessed the potential in vivo toxicity of the full human doses of the cancer vaccine candidates containing the WT1, p501, dHER2 or recPRAME tumor antigens combined with the AS15 immunostimulant in animal models. These repeated‐dose studies cover the schedules of immunization proposed in phase I and phase I/II clinical trials to patients with early metastatic disease or patients who are disease‐free after surgery. To this end, seven or 20 dose regimens were tested in rabbits and cynomolgus monkeys. Extensive histological, biochemical and immunological data are presented.
Materials and methods
Ethical statement and regulatory compliance
The study in rabbits (WT1 + AS15) was conducted in compliance with the OECD Principles of Good Laboratory Practices (GLP) (OECD, 1998), except for serology and bone marrow pathology evaluations. The study plan was in accordance with the Note for Guidance on Preclinical Pharmacological and Toxicological Testing of Vaccines (EMA, 1997).
Studies in monkeys (study 1, p501 + AS15; study 2, recPRAME + AS15; study 3, dHER2 + AS15; and study 4, p501 + AS15 [Table 1]) were conducted in compliance with CiToxLAB (Evreux, France) standard operating procedures and animal health regulations (The Council of the European Communities, 1986), under GLP conditions (Ministère de l'Emploi et de la Solidarité, 2000; OECD, 1998; The Commission of the European Communities, 1999; The European Parliament and the Council of the European Union, 2004), except for the determination of PSA levels in study 1 (p501 + AS15), prostate size measurements and laboratory investigations in study 4 (p501 + AS15), serology (all studies) and immunohistochemistry (IHC) analyses in studies 1 and 4 for which GLP compliance was not claimed.
Table 1.
Study design and methodology (rabbits and monkeys)
| Study | Candidate vaccine | No. of injections | No. of study animals | Study groups | Sex | No. of animals/group/sex | Day killed | Schedule | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical signs | Local reactions (h post‐injection) | Body weight (day) | Food consumption | Ophthalmology (day) | ECG | ||||||||
| Study in rabbits | |||||||||||||
| WT1 + AS15 | 7 | 60 | Saline | Males/females | 10 males/10 females | 87/112 | 2×/day | 3, 24, 48 | –4, 0, 3, 7 + weekly | daily | Pre‐injection + 87 + 108 | NP | |
| AS15 | |||||||||||||
| WT1 + AS15 | For subgroups: 5 males/5 females | ||||||||||||
| Studies in monkeys | |||||||||||||
| 1 | p501 + AS15 | 7 | 10 | Saline | Males | 5 | 88 | 1×/day | Pre‐injection | 1 + weekly + 87 | daily | Pre‐injection + 86 | Pre‐injection + 1 + 85 |
| p501 + AS15 | 1, 3, 24 | ||||||||||||
| 2 | recPRAME + AS15 | 7 | 20 | Saline | Males/females | 10: 5 males/5 females/group | 88/113 | 2×/day | 3, 24 | 1 + weekly | daily | Pre‐injection + 85 + 112 | Pre‐injection + 1 + 85 |
| recPRAME + AS15 | |||||||||||||
| Saline | |||||||||||||
| 3 | dHER2 + AS15 | 20 | 18 | AS15 | Females | 6 | 270/360 | 1×/day | Pre‐injection 3, 24, 48 | 1 + weekly + 86 + 87 | NE | Pre‐injection + 85 + 169 + 267 | Pre‐injection + 1 + 85 + 169 + 267 + 358 |
| dHER2 + AS15 | |||||||||||||
| 4 | p501 + AS15 | 20 | 18 | Saline | Males | 6 | 270/360 | 1×/day | Pre‐injection | Weekly until day 154 and biweekly thereafter | daily | Pre‐injection + 85 + 176 + 267 | Pre‐injection + 1 + 85 + 169 + 267 + 359 |
| AS15 | |||||||||||||
| p501 + AS15 | 1, 3, 24 | ||||||||||||
NE, not evaluated; no, number; NP, not performed; pre‐injection, before the first injection.
The designs of the studies conducted in monkeys were developed in accordance with the note for guidance on preclinical pharmacological and toxicological testing of vaccines, note for guidance on repeated dose toxicity, note for guidance on non‐clinical local tolerance testing of medicinal products and International Conference on Harmonisation Guideline S4A (EMA, 1997, 1999, 2000, 2001).
Study design
Study in rabbits
The study in rabbits was conducted at TNO Quality of Life Laboratories (Zeist, the Netherlands). Immune responses and bone marrow pathology (non‐GLP conditions) were evaluated at GSK Laboratories (Rixensart, Belgium). The New Zealand White albino rabbit was chosen as an animal model, as this non‐rodent species is commonly accepted by regulatory authorities for non‐clinical toxicity evaluation of vaccines. Initially, rabbits were randomly allocated to three groups of 20 animals (10 males and 10 females); the study was part of a larger one, including two more groups tested for different vaccines. Each group was further divided into two subgroups that were killed 3 or 28 days (4‐week recovery period) after the last injection (Table 1). Rabbits received seven injections of AS15 or WT1 + AS15. As repeated administration of a vaccine may result in an increasingly pronounced immune response, the number of administrations in the toxicity study should exceed the number planned for human administration to ensure confidence in the safety of the dosing schedule (Forster, 2012). Thus, we applied the “N + 1” rule, where at least one more administration is given in the toxicity study (seven injections) than in the proposed clinical scheme, which consist of successive cycles of a maximum of six injections administered at 3‐week intervals (NCT01220128). The 2‐week interval between the injections was aligned with the EMA and WHO guidelines for non‐clinical safety assessment of vaccines and adjuvants (EMA, 1999, 2005; WHO, 2003), and was still consistent with the 3‐week interval proposed in the clinical schedule (NCT01220128).
Studies in monkeys
The studies in monkeys were conducted at CiToxLAB. The cynomolgus monkey is an animal model well accepted by regulatory authorities, which was chosen because monkeys present the highest degree (>90%) of sequence homology among other species, and a similar pattern of expression with their human orthologs; thus, human tumor and self‐antigens can be tested in this animal model, allowing for assessment of potential immune‐ and product‐related toxicities. In addition, CiToxLAB maintains a historical reference database in which in vivo parameters, clinical pathology and histopathology in the cynomolgus monkey are available from all control animals used in previous studies at the laboratory, allowing for comparability regarding normal and baseline values. Furthermore, a full human dose of a given product can be injected in this species.
In study 1, 10 sexually mature male monkeys were allocated to groups using a computerized stratification procedure ensuring similar average body weight in each group. Monkeys received injections of saline (control group) or p501 + AS15 (treatment group) (Table 1). After the last injection, animals were kept for a 3‐day observation period.
In study 2, 20 purpose‐bred monkeys were allocated using a manual randomization procedure to two groups that received injections of saline (control group) or recPRAME + AS15 (treatment group) (Table 1). At the end of the treatment period (3 days after the last injection), the first three monkeys/sex/group were killed, while the remaining two monkeys/sex/group were killed after a 28‐day treatment‐free period.
In studies 3 and 4, 18 female (study 3) or male (study 4) monkeys were allocated based on clinical and laboratory examinations to receive injections of saline (control groups), AS15 alone (AS15 groups) or, in study 3, dHER2 + AS15 or, in study 4, p501 + AS15 (Table 1). At the end of the treatment periods, two monkeys per group were kept for a 13‐week treatment‐free period to evaluate the reversibility of potential toxic effects, while the remaining four monkeys per group were killed.
Studies 1 and 4 were conducted in males only as p501 + AS15 is intended to treat prostate cancer; study 3 was conducted in females only as dHER2 + AS15 is intended for the treatment of breast cancer. The number of injections was based on the “N + 1” rule mentioned above. In studies 1 and 2, monkeys received seven injections, reflecting single clinical cycles of six doses of p501 + AS15 (NCT00148928) or PRAME + AS15 (NCT01149343), administered every 2 weeks. The 20 injection schedule in studies 3 and 4 reflected the maximal human clinical exposure, with three cycles of six doses of dHER2 + AS15 (a total of 18 doses) (Hamilton et al., 2012), or three cycles of six, six and four doses of p501 + AS15 (a total of 16 doses) (NCT00148928), administered every 2 weeks. Furthermore, the repeated administration design mimicked the clinical procedure, in which some muscles receive multiple injections over time and some only unique injections, allowing for the assessment of the evolution of lesions induced at the injection sites with time.
Test items
Single candidate vaccine doses consisted of 200 µg of the recombinant WT1 protein, 100 µg of recombinant CPC‐p501 protein (p501), 500 µg of recombinant ProteinD‐PRAME‐His (recPRAME) or 500 µg recombinant and truncated dHER2 protein, with a fixed dose of the AS15 immunostimulant, containing MPL (GSK Vaccines, Rixensart, Belgium), QS‐21 (Quillaja saponaria Molina, fraction 21; Antigenics Inc., a wholly owned subsidiary of Agenus Inc., Lexington, MA, USA), CpG 7909 synthetic oligodeoxynucleotides containing unmethylated CpG motifs, and liposome (50 µg MPL, 50 µg QS‐21 and 420 µg CpG 7909).
The control item was a sterile isotonic saline solution (0.9% NaCl) (Belapharm GmbH& Co. KG, Germany [study in rabbits]; Fresenius, Sèvres, France [studies in monkeys]).
Experimental animals
Young adult New Zealand White albino rabbits were obtained from Centre Lago (France).
Cynomolgus monkeys (Macaca fascicularis) were obtained from approved suppliers from the Netherlands (study 1) or Mauritius (studies 2, 3 and 4). The initial age and body weight of the study animals are indicated in Table 2.
Table 2.
Study animals
| Study | Candidate vaccine | Initial age | Initial body weight range or mean (range) |
|---|---|---|---|
| Study in rabbits | |||
| WT1 + AS15 | 12 weeks | 2.3–2.6 kg | |
| Studies in monkeys | |||
| 1 | p501 + AS15 | 3–4 years | 3 kg (2.1–3.6 kg) |
| 2 | recPRAME + AS15 | At least 2 years | Males: 3.3 kg (2.5–4.2 kg) |
| Females 2.8 kg (2.5–3.0 kg) | |||
| 3 | dHER2 + AS15 | At least 2 years | 2.8 kg (2.4–3.3 kg) |
| 4 | p501 + AS15 | At least 4 years | 5.1 kg (4.2–5.9 kg) |
Housing and husbandry conditions are described in Supplementary materials (Supplementary Method S1).
Treatment and administration
In all studies, each dose (0.5 ml per animal per injection) of the tested vaccine corresponded to one full human dose. The intramuscular route was selected to mimic the intended route of administration in human therapeutic use.
In rabbits, an approximately 1.3 cm deep injection was performed in various muscles using a 23 gauge needle with a limiting device (Supplementary Table S1).
In monkeys, injections were administered in various muscles using a single use sterile plastic syringe fitted with a fine sterile single use needle (25–26 gauge; approximately 10–12 mm depth). Each site was clearly identified using an indelible pen (studies 1, 3 and 4) or by a tattoo (study 2). In studies 1 and 2, the injection sites were rotated, and only a single injection was given in each site, whereas in studies 3 and 4, both single and multiple injections were given (Supplementary Tables S2 and S3).
Clinical examinations
The schedule for evaluation of clinical signs and local reactions is indicated in Table 1. Rabbits showing abnormal clinical signs were subjected to a full physical examination. In monkeys, a detailed clinical examination of each animal was carried out once a week until the end of the study. Mortality and signs of morbidity were evaluated at least twice a day.
Skin reactions at the injection sites were recorded for each animal according to the following scale:
Erythema: 0: no erythema, 1: very slight (barely perceptible), 2: well defined, 3: moderate to severe, 4: severe (beet redness).
Edema: 0: no edema, 1: very slight (barely perceptible), 2: slight (edges of area well defined by definite raising), 3: moderate (raised approximately 1 mm), 4: severe (raised > 1 mm and extending beyond area of exposure).
If skin reactions were noted 24 h post‐injection, additional evaluations were repeated every 24 h until the reaction is no longer visible. Any other lesions (such as abscesses, necrosis or local inflammation) were recorded.
Body weight and food consumption were evaluated according to schedules indicated in Table 1.
Ophthalmology
In rabbits, ophthalmological examinations were performed in subgroup 2 using a direct ophthalmoscope. All visible structures of the eyes were examined. In monkeys, ophthalmological examinations were performed on all animals. Monkeys were tranquilized by an intramuscular injection of ketamine hydrochloride (Imalgène®; Mérial, Lyon, France), after which pupillary light reflexes were evaluated. The pupils were then dilated with tropicamide (Mydriaticum®; Merck Sharp & Dohme‐Chibret, Paris, France) and the appendages, optic media and fundus were examined by indirect ophthalmoscopy (Oméga 100; Heine, Herrsching, Germany). The anterior segment and the lens were also examined using a slit‐lamp biomicroscope.
Blood sampling
The blood sampling schedules for the assessment of hematology/biochemistry parameters, antibody responses and, for study 1 only, PSA and testosterone levels are shown in Supplementary Tables S1–S3.
In rabbits, blood was collected from the ear artery in heparinized tubes or tubes containing ethylenediaminetetraacetic acid (2 ml of blood) or citrate (1.8 ml of blood).
In monkeys, blood samples (approximately 3 ml) were taken from a peripheral vein without anesthesia and collected into tubes containing an appropriate anticoagulant. Before blood sampling, monkeys were fasted for an overnight period of at least 14 h. For the assessment of PSA levels (study 1), blood samples (approximately 2 ml) were taken into a tube without anticoagulant.
Before the analysis of PSA and testosterone levels, sera were prepared and stored frozen. The analyses were performed at Ecole Nationale Vétérinaire de Lyon, Marcy L'Etoile, France. PSA and testosterone levels in the sera were determined by radioimmunoassay using DSL (GLP compliant) and Beckman Coulter (GLP compliance not claimed) reagents respectively.
Blood hematology/biochemistry parameters
Using routine procedures, the following hematology and biochemistry parameters were assessed in all studies:
Hematology: erythrocytes, hemoglobin (Hb), mean and packed cell volume, mean cell Hb and mean cell Hb concentration, thrombocytes, white blood cells (WBC), differential WBC count with cell morphology (neutrophils, eosinophils, basophils, lymphocytes, monocytes), reticulocytes, prothrombin time, activated partial thromboplastin time (APTT) and fibrinogen.
Biochemistry: glucose, alkaline phosphatase, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), γ‐glutamyl transferase, total protein, albumin, albumin/globulin (A/G) ratio, urea, creatinine, creatine kinase, lactate dehydrogenase, total bilirubin, cholesterol, triglycerides, phospholipids, calcium, sodium, potassium, chloride and inorganic phosphate.
Pathology: necropsy, tissue processing and examination
Study in rabbits
Rabbits were anesthetized by an intravenous injection with Nembutal (60–120 mg kg–1) and then killed by opening the abdominal aorta.
For macroscopic examination of the injection sites, the central area of the thigh muscle was incised at the site of injection. For gross findings, the site was sampled and preserved in a neutral aqueous phosphate‐buffered 4% solution of formaldehyde. If no gross findings were present, the adjacent muscle tissue was inspected for gross findings and, if present, sampled and preserved. Organs and tissues weighed and examined are specified in Supplementary Table S4.
Only the right calf muscle was examined microscopically to assess the development of the local reaction over time. Selected tissues were embedded in paraffin wax, sectioned at 5 µm and stained with hematoxylin and eosin. Three areas of the preserved right calf muscle, central and adjacent left and right areas, were processed and examined macroscopically for gross findings. Tissues and organs not submitted to histopathological examination were kept in formaldehyde.
Studies in monkeys
After at least 14 h fasting, monkeys were tranquilized by intramuscular injection of ketamine hydrochloride and killed by an intravenous injection of thiopental sodium followed by exsanguination.
In monkeys, a complete macroscopic post‐mortem examination was performed on all animals. This included examination of the external surfaces, all orifices, the cranial cavity, the external surface of the brain and spinal cord, the thoracic, abdominal and pelvic cavities with their associated organs and tissues and the neck with its associated organs and tissues. The organs of the monkeys specified in Supplementary Table S4 were weighed wet as soon as possible after dissection. Organ weight/body weight ratio immediately before killing was calculated.
The tissues were preserved in 10% buffered formalin (except for the eyes with optic nerve, which were fixed in Davidson's fixative, and the testes and epididymides, which were preserved in Bouin's fluid).
All tissues required for microscopic examination were embedded in paraffin wax, sectioned (4 µm) and stained with hematoxylin and eosin (except for testes and epididymides, which were stained with hematoxylin/periodic acid–Schiff). In studies 1 and 4, paraffin‐embedded or blank superfrost slides were prepared from organs specified in the section “Immunohistochemistry (studies 1 and 4)” for evaluation of the p501 protein expression by IHC.
A microscopic examination was performed on all tissue or organ samples listed in Supplementary Table S4. For the injection sites, in studies 1 and 2, only the last injection site (left biceps) was examined microscopically on day 85 (study 1) or on day 88 and 113 (study 2); in studies 3 and 4, the left biceps (multiple injection site) and all single injection sites were examined on days 1, 85, 169 and 267.
Serology (non‐good laboratory practice)
WT1‐specific (rabbits), p501‐specific (monkeys, studies 1 and 4), PRAME‐specific (monkeys, study 2) and dHER‐specific (monkeys, study 3) antibodies were measured by an enzyme‐linked immunosorbent assay (ELISA), using the respective antigen as coating antigen.
At each assessment time point, antibody geometric mean titers were calculated on individual sera (start dilution 1 : 5000 for rabbits and 1 : 100 or 1 : 1000 for monkeys, followed by serial dilution 2 : 2) referring to a standard curve established with a standard serum and using a validated internal control (pooled sera collected after injections of the given product in the same animal species). Seroconversion was defined with a threshold expressed in optical density (OD). This threshold represents three times the mean of OD obtained with sera of animals only receiving saline at each time point. The seroconversion was calculated on sera at the dilution of 1 : 2000 for p501 and PRAME and 1 : 100 for dHER2. For WT1, the limit of quantification of the test was chosen as threshold; because the calculated threshold was below the limit of quantification, the dilution of 1 : 40 000 was used to calculate seroconversion. OD was measured using a microplate reader (Bio‐Rad Laboratories, Berkeley, CA, USA) connected to a computer. Data were captured with the SoftMaxPro software and analyzed.
Additional assessments (studies in monkeys)
Electrocardiography
Electrocardiographic (ECG) examinations were performed on all monkeys at pre‐injection and approximately 1 h (±15 min) post‐injection as specified in Table 1. Before examinations, animals were not anesthetized but were kept awake in a restraining chair during the examination, or were tranquilized (study 2) by intramuscular injection of ketamine hydrochloride (Imalgène®; Mérial). ECG examinations were performed using a Delta 3 Plus Cardioline (Care Systems, Saint‐Germain‐en‐Laye, France) fitted with standard leads I, II and III.
Immunohistochemistry (studies 1 and 4)
Immunohistochemical evaluations of the expression pattern of the monkey p501 protein in thyroid, colon, prostate and, in study 4 only, pancreas, stomach, spinal cord, testes and brain (cerebellum and cortex) tissue samples were performed under non‐GLP conditions. p501 is a differentiation antigen for the prostate; the other organs were evaluated as negative controls.
Five µm thick paraffin‐embedded sections (study 1) or 6 µm thick blank superfrost slides (study 4) were incubated with a polyclonal antibody raised against the human p501 protein (1 : 500). In addition, in study 4, non‐specific immunostaining was performed using a polyclonal rabbit antibody raised against the Borrelia burgdorferi outer surface protein A (OspA) (anti‐OspA; 1 : 300). Antibody binding was detected using a biotinylated antirabbit mouse monoclonal antibody (Sigma‐Aldrich, St. Louis, MO, USA) and streptavidin‐horseradish conjugate (Zymed, San Francisco, CA, USA). 3,3′‐diaminobenzidine was used as chromogen (DAB detection kit; Invitrogen, Carlsbad, CA, USA). Slides were counterstained with hematoxylin.
Statistics
Study in rabbits
Body weight, body temperature and clinical pathology data were analyzed using one‐way analysis of covariance, with corresponding pretreatment values as covariates, using Bartlett's test and Shapiro–Wilk's test, and, for data showing non‐homogeneity of group means, group t‐tests or the Mann–Whitney rank test. Food consumption data were analyzed using Dunnett's multiple comparison test. The statistical analyses were performed using Provantis software (version 6.5). Incidences of histopathological changes were analyzed using Fisher's exact probability test. Continuous and semicontinuous data were reported as arithmetic means and SD.
Studies in monkeys
Statistical analyses of body weight, body temperature, ECG, hematology and blood biochemistry data were performed using Citox software (versions D.3 to D.6 covering the period of the studies). PathData software (version 6.2b5) was used for the statistical analysis of organ weight data.
Results
Study in rabbits
Clinical observations
No distinct treatment‐related clinical signs were observed during the study period. Occasionally, due to a punctured subcutaneous blood vessel upon injection, a hematoma at the injection site was observed in rabbits from all three groups. Other clinical signs occasionally observed were considered unrelated to treatment.
Four hours after the seventh injection, the rectal body temperature was statistically higher in males from the WT1 + AS15 group, compared with the control group (39.3 °C vs. 38.8 °C; P < 0.01). There was no effect of treatment with AS15 or WT1 + AS15 on body weight, food intake or ophthalmoscopy parameters.
Blood hematology and biochemistry parameters
Following the first and the seventh injections, a transient increase of fibrinogen levels, total protein, decreased albumin (females only) and decreased A/G ratios were observed in rabbits from the AS15 and WT1 + AS15 groups as compared to controls (Fig. 1).
Figure 1.
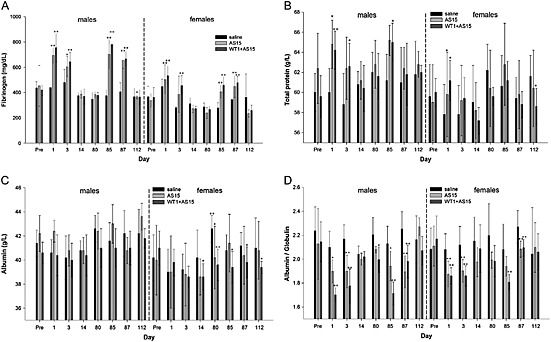
Pre‐ and post‐injection blood hematology/biochemistry parameters: (A) fibrinogen, (B) total protein, (C) albumin and (D) albumin/globulin ratios of male and female rabbits from the control, AS15 and WT1 + AS15 groups. The time points of assessment are indicated: pre, pre‐treatment; days post‐injection, refer to Supplementary Table S1 for correspondence. The error bars represent SDs. Statistically significant differences are shown as *P < 0.05 or **P < 0.01; two‐sided ANOVA.
Pathology
Macroscopic examination did not reveal any significant findings and no treatment‐related changes in organ weights were observed. Three days after the last injection, microscopic examination of the injection site revealed a widespread mononuclear cell infiltrate in rabbits from the WT1 + AS15 and AS15 groups; 28 days after the last injection, the inflammation was diminished in both groups.
Disintegration of the epimysium and fibrosis were observed in the WT1 + AS15 and AS15 groups. Necrosis and mineralization, associated with the inflammation, were only observed in the WT1 + AS15 group.
Three days after the seventh injection, activated popliteal lymph nodes and lymphadenitis in the injected right hind leg were observed in rabbits from the WT1 + AS15 group. This finding was considered related to the inflammation.
Serology
A 100% seroconversion rate for WT1‐specific antibodies was observed in rabbits from the WT1 + AS15 group. No difference in seroconversion was observed between male and female rabbits. In rabbits from the control and the AS15 groups, WT1‐specific antibodies were not detected at any time point. High WT1‐specific antibody titers were detected 3 and 28 days after seven injections of WT1 + AS15 in both male and female rabbits (Fig. 2).
Figure 2.

WT1‐specific antibody response in male and female rabbits injected with WT1 + AS15. The red bars represent geomean values. The time points of assessment are indicated: pre, pre‐treatment; day 87, 3 days post seventh injection; day 112, 28 days post seventh injection. Ab, antibody; EU/ml, ELISA units/ml.
Studies in monkeys
Mortality, systemic toxicity and clinical signs
There were no morbidities or unscheduled deaths during the studies. In study 1 (p501 + AS15), no clinical signs or systemic toxicity were recorded. In study 2 (recPRAME + AS15), the incidence of clinical signs was similar between the control and treated animals (alopecia, scabs, brownish vomit, hand/forearm with increased size, nostril with reddish liquid discharge); thus, the relationship to the treatment with recPRAME + AS15 was excluded. In studies 3 (dHER2 + AS15) and 4 (p501 + AS15), no treatment‐related systemic clinical signs were noted. Soft or liquid feces were noted as transiently occurring in all groups; in study 4, the incidence was higher in the control group, in which five of six animals were affected during the treatment period and, occasionally, during the treatment‐free period. However, soft or liquid feces are commonly seen in untreated gang housed monkeys, and thus, this event was considered to be unrelated to treatment. In study 4, where necessary, the animals were treated for liquid feces with nifuroxazide (Panfurex®; Bouchara‐Recordati, Saint‐Victor, France) or diosmectite (Smecta®; Ipsen, Dreux, France). Other clinical findings were alopecia, scabs, wounds and erythema (study 3) but were incidental and unrelated to treatment, as they appeared in all study groups. Vomiting (study 4) was noted in one control, one AS15 and three monkeys from p501 + AS15 group on day 91, but was considered secondary to the anesthesia with Imalgène (Mérial), required for the ophthalmology examinations and measurement of testicular and prostate sizes, as it was observed in all groups on one occasion only.
Local reactions
No local reactions were recorded in study 1 (p501 + AS15).
Moderate findings, considered related to the recPRAME + AS15 treatment, were observed at the injection sites during the treatment period in study 2, which were: erythema seen in four of five males and five of five females at one or several injection sites, most often on the day after injection and lasting 1–2 days, and edema in two of five males and five of five females at three of seven injection sites. Hematomas, observed in the control and treated monkeys with similar incidence, were most probably caused by the injection procedure and were not considered treatment‐related.
No marked skin reactions were recorded at the single or multiple injection sites in the control monkeys in study 3. In contrast, very slight to severe erythema and very slight to slight edema were noted in the AS15 and dHER2 + AS15 groups throughout the study, with a higher severity and incidence at sites of repeated injections. Erythema was observed up to 8 days post‐injection and edema up to 7 days post‐injection. No skin reactions were observed at the end of the treatment‐free period.
No treatment‐related signs of local intolerance at the injection sites were noted in study 4. Very slight erythema was observed in all groups after the injection in week 15. In the p501 + AS15 group, one monkey presented alopecia on the head (week 2), and on forelimbs and shoulders (week 12), and one, a slight alopecia on the head (week 5) that persisted up to the study end. These observations are often seen in untreated monkeys, and were considered of no toxicological importance. These two monkeys also had scabs and wounds on the shoulders or forearms observed for a few days during the first half or at the end of the study period. Nevertheless, this was not observed at the injection sites, and similar findings were noted in the control and AS15 groups. Thus, a relationship to treatment with p501 + AS15 was unlikely. These lesions were treated with povidone–iodine or aluminum powder to avoid any infection.
Body weight
In study 1 (p501 + AS15), the mean body weight gain of all treated monkeys over the study period was 33% lower (+0.4 kg) than that of the controls (+0.6 kg). Although this difference is likely to be within the normal limits, a relationship to the treatment could not be excluded.
Body weights were not affected by the treatments in studies 2 (recPRAME + AS15) and 3 (dHER2 + AS15).
In study 4, over the complete treatment period (week 1–39), the mean body weight gain of the AS15 and p501 + AS15‐treated monkeys was 41% and 35% lower, respectively (+1.0 kg and +1.1 kg) than that of the control monkeys (+1.7 kg); thus, a relationship to the treatment with AS15 or p501 + AS15 was not excluded. No differences in body weight gain were observed during the treatment‐free period, indicating reversibility.
Body temperature
In study 1, there was no effect of treatment with p501 + AS15 on body temperature.
In study 2, 24 h after the first injection, the mean rectal temperature of the recPRAME + AS15‐treated males and females was statistically significantly higher than in the controls (39.3 °C vs. 38.4 °C in males [P < 0.01] and 39.1 °C vs. 38.4 °C in females [P < 0.05]). This temperature increase was attributed to the test item treatment. At the end of the treatment period, no statistically significant differences were noted.
A statistically significantly higher body temperature in the AS15 and dHER2 + AS15 groups (study 3) than in controls was recorded at several time points (Fig. 3). Of note, a statistically significantly higher temperature in the AS15 and dHER2 + AS15 groups compared to control was recorded 1 day before the first injection (39.0 °C and 39.3 °C respectively, vs. 38.5 °C in the control group); this was considered fortuitous. Hyperthermia at 4 or 24 h post‐injection was observed in the AS15 and dHER2 + AS15 groups, and was thus considered related to injection of these test items. The body temperatures measured at the end of the treatment‐free period were similar in all groups.
Figure 3.
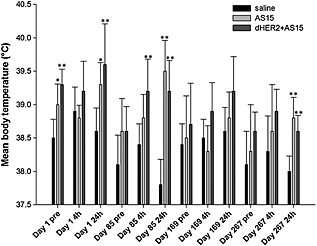
Pre‐ and post‐injection body temperature in monkeys injected with saline, AS15 and dHER2 + AS15 (study 3; dHER2 + AS15). The time points of assessment are indicated: Day 1, first dose administration; Day 85, seventh dose administration; Day 169, 13th dose administration; Day 267, 20th dose administration. Pre, before dose administration; 4h, 4 h after the administration; 24h, 24 h after the administration. Statistically significant differences are shown as *P < 0.05 or **P < 0.01; Dunnett's test.
In study 4 (p501 + AS15), no treatment‐related differences in body temperatures were noted during the treatment and treatment‐free periods.
Food consumption, electrocardiography and ophthalmoscopy
Food consumption was not considered affected by treatment with the tested vaccines in all studies.
One male from the p501 + AS15 group in study 4 showed ventricular premature complex arrhythmias before the seventh injection (day 85). As this was an isolated finding, not observed pre‐injection or in the control group, a relationship to treatment with p501 + AS15 was considered unlikely. No other treatment‐related effects were observed for ECG quantitative and qualitative parameters.
One female from study 3 (dHER2 + AS15 group) had hypopigmented fundus in both eyes before the first injection and on days 85, 169 and 267; as this was observed before the treatment start as well as post‐injection, it was considered unrelated to the study treatment. No other ophthalmological lesions were observed in any group.
Blood parameters
Hematology
In study 1 (p501 + AS15), the mean WBC count was statistically significantly higher in the treatment than in the control group on day 4 (15.82 vs. 12.51 × 109 l–1; P < 0.05). On day 88, mean neutrophil and monocyte counts were statistically significantly higher in the treatment group, compared to controls (6.55 vs. 2.58 × 109 l–1 [P < 0.01] and 0.69 vs. 0.38 × 109 l–1 [P < 0.05], respectively).
In study 2 (recPRAME + AS15), the mean neutrophil count, APTT and mean fibrinogen concentration were statistically significantly higher in males and females from the treatment group compared to the controls at several post‐injection time points during the treatment period (Fig. 4). At the end of the treatment‐free period, no statistically significant differences were observed, indicating that these changes were transient.
Figure 4.
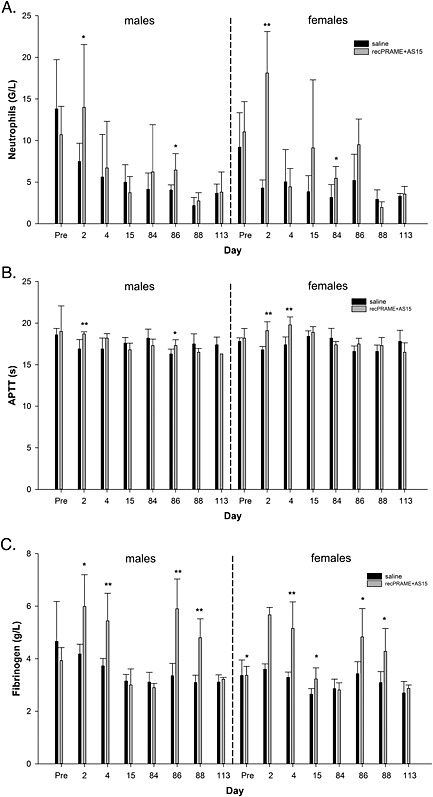
Pre‐ and post‐injection blood hematology parameters: (A) neutrophils, (B) APTT and (C) fibrinogen of male and female monkeys from the control and recPRAME + AS15 group (study 2; recPRAME + AS15). The time points of assessment are indicated: pre, pre‐treatment; days post‐injection, refer to Supplementary Table S2 for correspondence. Statistically significant differences are shown as *P < 0.05 or **P < 0.01; Dunnett's test. APTT, activated partial thromboplastin time; G/L, 109 l–1.
In study 3 (dHER2 + AS15), mean fibrinogen levels were statistically significantly higher, and erythrocyte counts, packed cell volume and Hb concentrations were statistically significantly lower in the AS15 and dHER2 + AS15 groups at several post‐injection time points (Fig. 5). These changes were considered treatment‐related. There were no significant differences between the groups at the end of the treatment‐free period (day 358).
Figure 5.
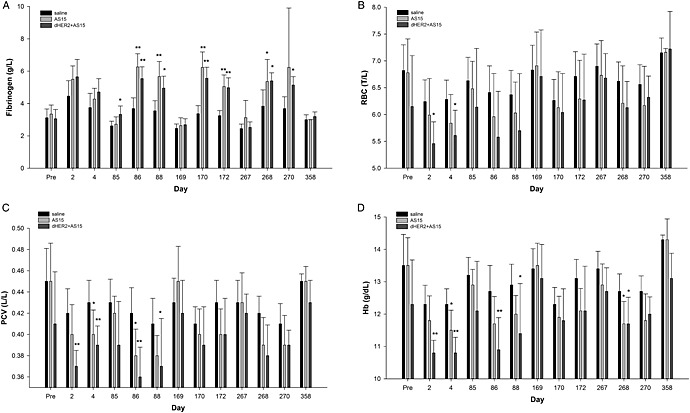
Pre‐ and post‐injection blood hematology parameters: (A) fibrinogen, (B) RBC, (C) PCV and (D) Hb of female monkeys from the control, AS15 and dHER2 + AS15 groups (Study 3: dHER2 + AS15). The time points of assessment are indicated: pre, pre‐treatment; days post‐injection, refer to Supplementary Table S3 for correspondence. Statistically significant differences are shown as *P < 0.05 or **P < 0.01; Dunnett's test. Hb, hemoglobin; PVC, packed cell volume (hematocrit); RBC, red blood cells; T/L, 1012 l–1.
A significant increase in the fibrinogen concentration was noted 1 and 3 days post‐injection in the AS15 and p501 + AS15 groups of study 4 (Fig. 6). The WBC counts, associated with increased neutrophil and sometimes eosinophil and monocyte counts, were also increased 1 day post‐injection, whereas the lymphocyte counts were decreased, which was predominantly observed in the p501 + AS15 group (Fig. 6). Three days post‐injection, these values were generally similar to those of the control animals. There were no differences between groups at the end of the treatment‐free period.
Figure 6.
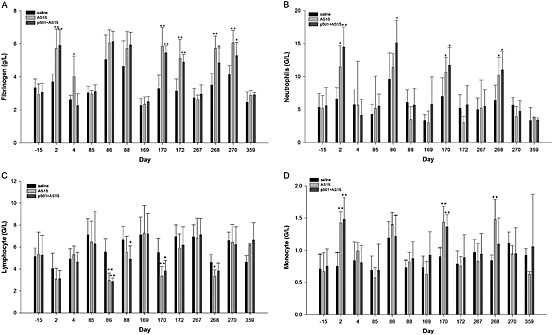
Pre‐ and post‐injection blood hematology parameters: (A) fibrinogen, (B) neutrophil, (C) lymphocyte and (D) monocyte levels of male monkeys from the control, AS15 and p501 + AS15 groups (study 4; p501 + AS15). The time points of assessment are indicated: –15, 15 days pretreatment; days post‐injection, refer to Supplementary Table S3 for correspondence. Statistically significant differences are shown as *P < 0.05 or **P < 0.01; Dunnett's test. G/L, 109 l–1.
Biochemistry
In study 1, the mean A/G ratio was statistically significantly lower (P < 0.05) in the p501 + AS15 than in the control group on days 2, 4, 85, 86 and 88 (A/G was 1.05, 1.02, 1.07, 0.95 and 0.86 in the p501 + AS15 group vs. 1.23, 1.22, 1.25, 1.16 and 1.15, respectively, in the control group). As this decrease was observed consistently in all treated monkeys and was occasionally statistically significant, it was considered treatment‐related (inflammatory process). A decrease in the mean inorganic phosphorus level was noted on day 2 (1.16 vs. 1.85 mmol l–1 pre‐dose [–37.3%] and 1.38 mmol l–1 in controls [–15.9%]), and on day 86 (1.15 vs. 1.85 mmol l–1 pre‐dose [–37.8%] and 1.53 mmol l–1 in controls [–24.8%]). In addition, on days 86 and 88, the mean ASAT and ALAT levels were slightly higher than that of controls. The changes in inorganic phosphorus, ASAT and ALAT were transient over the study and mainly attributed to one or two animals; thus, they were not considered of toxicological relevance.
There were no treatment‐related differences in blood biochemistry parameters in studies 2 and 4.
In study 3, during the first half of the treatment period, transiently and minimally statistically higher bilirubin, urea and creatinine levels were observed in the AS15 and dHER2 + AS15 groups, compared to control. Thus, the increases in these biochemistry parameters were considered related to the AS15 or dHER2 + AS15 injections. The values of these parameters returned to the control levels within 3 days post‐injection or by the next sampling time point. In particular, bilirubin levels increased compared to controls 1 day after the second and third injection in the AS15 group (10 and 9 µmol l–1 vs. 5 and 6 µmol l–1, respectively, in controls; P < 0.01). Creatinine levels also increased in the AS15 group, but only 1 day after the second injection (83 vs. 73 µmol l–1 in control; P < 0.01). In the dHER2 + AS15 group, bilirubin, urea and creatinine increased 1 day after the first injection compared to controls (10 vs. 7 µmol l–1 for bilirubin [P < 0.05]; 6.1 vs. 4.5 mmol l–1 for urea [P < 0.01] and 85 vs. 77 µmol l–1 for creatinine [P < 0.01]). There were no differences between groups at the end of the treatment‐free period.
Prostate‐specific antigen and testosterone levels (study 1; p501 + AS15)
The PSA levels in the control and p501 + AS15‐treated monkeys were unchanged at the end of the study period. One control and one treated male showed a marked decrease in testosterone levels (from 18.8 nmol l–1 pre‐injection to 4.4 nmol l–1 in week 13, and from 43.4 nmol l–1 pre‐injection to 2.1 nmol l–1 in week 13, respectively). No other marked changes in testosterone levels were observed in other monkeys from either group at the end of the study period.
Pathology
No vaccine‐related effects on organ weights were observed in study 1 (p501 + AS15). The mean weights of prostates and seminal vesicles were slightly higher in the control than in the p501 + AS15 group (1.16 g vs. 0.74 g and 3.23 g vs. 2.19 g, respectively). However, these differences were largely related to variations in sexual maturity, as monkeys in the control group were generally more mature than in the treatment group. Thus, this was not considered to be of toxicological importance. Macroscopic post‐mortem examination did not reveal any treatment‐related effects. Microscopic examination of the injection site (left biceps) revealed myodegeneration, necrosis, sacrolemmal nuclei proliferation, interstitial inflammatory cells and interstitial fibrosis in one control and in four of five treated monkeys. The higher incidence and severity of these histopathological changes in the treated monkeys were considered related to treatment with p501 + AS15. No treatment‐related microscopic systemic findings were noted. There was no evidence of lymphoid cell infiltrate in any organ, including the prostate.
In study 2 (recPRAME + AS15), mean weights of axillary lymph nodes were higher in the treated males at the end of the treatment period (absolute weight: +139%; relative weight: +149% vs. control) and females (absolute weight: +31% and relative weight: +34% vs. control). The increased weights correlated with increased (unilateral or bilateral) paracortex size or cellularity observed microscopically, and with enlarged axillary lymph nodes at necropsy (Table 3). These changes were probably related to the antigenic stimulation or muscular inflammation secondary to the seventh injection (left biceps). At the end of the treatment‐free period, mean absolute and relative weights of iliac, axillary, popliteal and inguinal lymph nodes were higher (up to +120%) in males and females from the treatment group, compared to controls. In addition, mean weights of the cervical lymph nodes were higher in males from the treatment group compared to the controls. Given the low numbers of monkeys and the absence of microscopic correlates, these variations were considered incidental and unrelated to the recPRAME + AS15 injections. No other recPRAME + AS15‐related effects on the organ weights were recorded. Macroscopic post‐mortem examination revealed enlarged iliac, axillary and popliteal lymph nodes in one male, and enlarged axillary lymph nodes in one female from the treatment group. This enlargement correlated with increased paracortex size and cellularity in the axillary lymph nodes and with increased germinal center size and numbers, or increased plasma cell numbers in the other lymph nodes. Enlarged lymph nodes are commonly seen in control cynomolgus monkeys and were recorded in the mandibular lymph nodes of one‐third of males in the current study. A relationship to recPRAME + AS15 injections could not be excluded for the axillary lymph nodes draining the last injected site (left biceps), whereas enlargement of the other lymph nodes was considered incidental. Other macroscopic findings had no histological correlates or correlated with common histological findings in control monkeys, and were thus considered incidental. No treatment‐related macroscopic findings were recorded at the end of the treatment‐free period. Microscopic post‐mortem examination of the revealed interstitial inflammatory cell infiltrates composed of a mixture of mononuclear cells (macrophages, lymphocytes and plasma cells), often accompanied by interstitial edema, in all monkeys from the treatment group. Occasional interstitial hemorrhages and fibroplasia/fibrosis were also observed. In all males and two‐thirds of females from the treatment group, an increased paracortex size or cellularity was noted in the axillary lymph nodes (Table 3). This finding was probably related to the antigenic stimulation or muscular inflammation secondary to the seventh injection. At the end of the treatment‐free period, no microscopic findings in this injection site, and no significant changes in incidence or severity of the microscopic findings in the lymph nodes were observed in monkeys from the treatment group compared to control, indicating complete recovery.
Table 3.
Microscopic treatment‐related findings in monkeys injected with recPRAME + AS15 (n = 6; study 2) at the end of the treatment period
| Male (n = 3) | Female (n = 3) | |
|---|---|---|
| Left biceps brachii | ||
| Inflammatory cell infiltrate | ||
| grade 1 | 2 | – |
| grade 2 | 1 | 2 |
| grade 3 | – | 1 |
| Edema | ||
| grade 1 | 3 | 2 |
| Hemorrhage | ||
| grade 1 | 1 | 1 |
| Fibroplasia/fibrosis | ||
| grade 1 | 1 | – |
| Lymph nodes | ||
| Axillary lymph nodes | ||
| Increased paracortex size/cellularity | ||
| grade 2 | 1 | – |
| grade 3 | 2 | 2 |
| Iliac lymph nodes | ||
| Increased germinal center size/numbers | ||
| grade 1 | 1 | – |
| grade 2 | 1 | 1 |
| Increased paracortex size/cellularity | ||
| grade 3 | 1 | – |
| Increased plasma cell numbers | ||
| grade 2 | – | 1 |
| grade 3 | 1 | – |
| Inguinal lymph nodes | ||
| Increased germinal center size/numbers | ||
| grade 2 | 1 | 1 |
| grade 3 | 1 | 1 |
| Increased plasma cell numbers | ||
| grade 2 | – | 1 |
n, number of animals within the group. Severity was evaluated according to a 1–4 scale: 1 = minimal/very few/very small; 2 = slight/few/small; 3 = moderate/moderate number/moderate size; 4 = marked/many/large.
No relevant differences in the organ weights between the AS15 or dHER2 + AS15‐treated and the control monkeys were noted in study 3. Macroscopic post‐mortem examination did not reveal any significant findings. Microscopic examination of the injection sites revealed infiltration of inflammatory cells, mainly macrophages, in the aponeurosis of the left calf muscle (single injection site, day 267) 3 days before death, and was often accompanied by an eosinophilic material consistent with the injected material. This lesion, described as “aponeurosis: inflammatory cell infiltration” was found only in the monkeys from the AS15 (all) or dHER2 + AS15 (two of four) groups with a similar severity, and was thus considered to be treatment‐related. In one of four monkeys from the AS15 group, inflammatory cells in aponeurosis/fibrous fascia of the left biceps were noted on days 15, 57, 113, 155, 211 and 253. This finding is comparable to the epimysium observed in rabbits injected with AS15 or WT1 + AS15 (see section “Blood hematology and biochemistry parameters” on pathology). The fascia was thickened by fibrosis and numerous small vessels and a few mononuclear cells were noted. The lesion was chronic, minimal in severity. Myodegeneration/necrosis was noted at all injection sites in all monkeys, including controls.
In study 4 (p501 + AS15), higher mean prostate weights, absolute and relative to the body weight, were observed in male vaccine recipients compared to controls, while lower weights were observed in males from the AS15 group (Table 4). These findings correlated with those for prostate volume measurements. Specifically, the group mean (±SD) prostate weights were 3.26 ± 1.13, 1.94 ± 0.61 and 4.14 ± 2.32 g for the control, AS15 and p501 + AS15 groups, respectively, at the end of the treatment period, and the mean volumes were 3.61 ± 0.80, 2.43 ± 0.59 and 3.42 ± 1.13 cm3, in these groups respectively. However, the organ weight and volume differences between the control and AS15 groups or the control and p501 + AS15 groups were not statistically significant, and the number of monkeys evaluated in each group is low (n = 4). Furthermore, organ weights for male reproductive organs can be influenced by age, body weight or sexual maturity. At the end of the treatment period, the terminal prostate volume correlated well with the terminal body weight (Supplementary Table S5), suggesting that the apparent differences between control and treatment groups could be explained by the weight (size) of the animals in each group; thus, it seems unlikely that the observed values could be attributed to treatment with the AS15 or p501 + AS15 groups. In addition, epithelial cell hypertrophy was noted in the prostate from two of four monkeys from the p501 + AS15 group. In a single male, this lesion correlated with an increase in prostate weight; thus, a relationship to treatment could not be excluded. Nevertheless, this was not seen at the end of the treatment‐free period, indicating complete reversibility. As compared to controls, lower absolute and relative thymus and spleen weights were observed in the p501 + AS15 group, and lower absolute and relative thyroid weights were noted in the AS15 and p501 + AS15 groups (Table 4); thus, these findings are probably related to treatment with AS15 or p501 + AS15. At the end of the treatment‐free period, no differences in the prostate and spleen weights between the controls and p501 + AS15‐treated monkeys were noted, suggesting reversibility. Macroscopic examination revealed hematomas in subcutaneous tissue on the right and left front thighs and the left calf among males from the control and AS15 groups. These lesions were considered related to the injection procedure. At the end of the treatment‐free period, hematoma in subcutaneous tissue in the left calf was noted in one control male. This was considered related to the injection procedure and correlated well with microscopic findings. Microscopic examination of the injection sites revealed lesions (i.e. hemorrhage, perivascular neutrophilic infiltration, interstitial edema and interstitial fibrosis) in monkeys from all three groups at the end of the treatment and treatment‐free periods, which were considered related to injection procedure. In addition, in one male from the p501 + AS15 group, multifocal slight segmental chronic periarteritis was seen in both kidneys; the relationship to treatment remains unclear as this event was noted in only one of four monkeys from this group and is generally rarely observed in cynomolgus monkeys.
Table 4.
Absolute and relative organ weight differences (expressed in %) in monkeys from the AS15 and p501 + AS15 groups, as compared to controls (study 4)
| Group | ||
|---|---|---|
| AS15 | p501 + AS15 | |
| Liver | ||
| absolute | +2 | +3 |
| relative | +15* | +9 |
| Testes | ||
| absolute | –3 | +9 |
| relative | +8 | +18 |
| Prostate | ||
| absolute | –40 | +27 |
| relative | –32 | +36 |
| Spleen | ||
| absolute | –3 | –28 |
| relative | +12 | –24 |
| Thymus | ||
| absolute | +2 | –36 |
| relative | +22 | –29 |
| Thyroid | ||
| absolute | –24 | –22 |
| relative | –13 | –19 |
Dunnett's test based on pooled variances at 5% level.
Serology
In study 1, at day 88, a 100% seroconversion rate for p501‐specific antibodies was observed in the p501 + AS15 group and no seroconversion was observed in the control group.
In study 2, before the injections, all monkeys were seronegative for recPRAME‐specific antibodies. Following the fourth injection, no seroconversion was observed in the control monkeys (data not shown), while all monkeys injected with recPRAME + AS15 were seropositive, with mean titers reaching 33 293.5 EU ml–1. Antibody titers did not increase further, but remained high at 3 days and 1 month after the seventh injection (mean titer 35 194.6 and 26 081.5 EU ml–1, respectively) (Fig. 7A).
Figure 7.
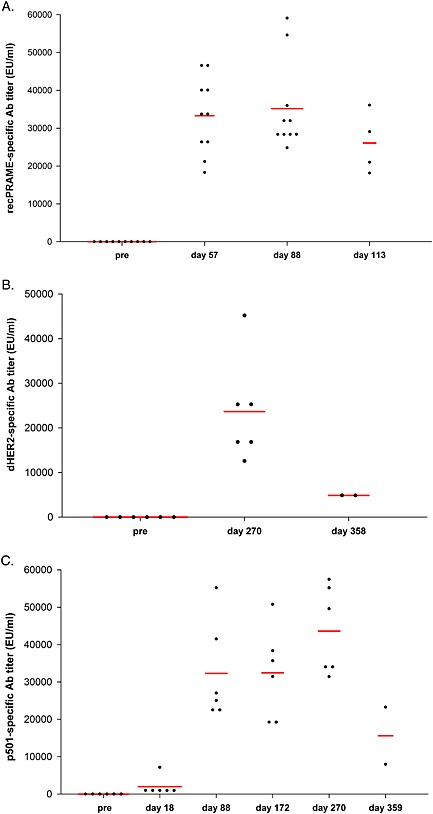
recPRAME‐, dHER2‐ and p501‐specific antibody response in monkeys injected with respectively recPRAME + AS15 (study 2), dHER2 + AS15 (study 3) or p501 + AS15 (study 4), expressed as antibody titers. pre, pre‐injection (group of 10 monkeys); Ab, antibody; EU, ELISA units. The red bars represent geomean values. The time points of assessment are indicated: pre, pre‐treatment; recPRAME + AS15: 14 post‐4, 14 days post fourth injection; 3 post‐7, 3 days post seventh injection; 28 post‐7, 28 days post seventh injection; dHER2 + AS15: day 270, post last injection; day 358, end of treatment‐free period; p501 + AS15: day 18, post second injection; day 88, post seventh injection; day 172, post 13th injection; day 270, post 20th injection and day 359, end of treatment‐free period.
A 100% seroconversion rate was observed for the dHER2‐specific antibodies in the dHER2 + AS15 group of study 3. No seroconversion was observed in the control and AS15 groups. In the dHER2 + AS15 group, high dHER2‐specific antibody levels were observed at 3 days after the last injection (day 270), and were still observed at the end of the treatment‐free period (day 358), although at lower levels (Fig. 7B).
No p501‐specific antibody response was observed before treatment in all groups in study 4 and no seroconversion was observed in the control and AS15 groups during the treatment period. A 100% seroconversion rate was observed in monkeys from the p501 + AS15 group already after the second injection and was maintained over time (the two monkeys analyzed 3 months after the last injection were still seropositive), and the p501‐specific antibody levels increased further after the seventh, 13th, and last injection (Fig. 7C).
Immunohistochemistry (studies 1 and 4; p501 + AS15)
The expression of p501 protein was only observed in mature prostates in study 1. The differences in p501 protein expression observed between the treatment vs. control groups were probably due to sexual immaturity rather than treatment effect. No p501 protein expression was observed in thyroid and colon (negative control).
In study 4, high p501 protein expression was observed in all prostate samples from all three groups (Fig. 8A). Compared to the control group, the expression of p501 in the prostate did not seem to be affected by repeated injections of p501 + AS15. A low p501 protein expression was observed in all pancreas samples (except for one monkey from the AS15 group) and was limited to the Langerhans cell islets or endothelial cell membranes from vessels (Fig. 8B). This immunostaining was considered p501‐specific, as no immunopositive response was observed when an irrelevant rabbit anti‐OspA antibody was used (Fig. 8C). No p501 expression was observed in the negative control samples (stomach and brain cortex [Fig. 8D,F] and spinal cord, thyroid, colon, testes and brain cerebellum [data not shown]).
Figure 8.
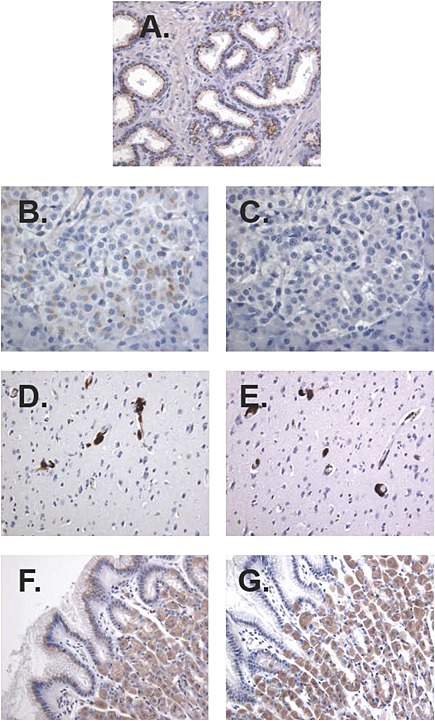
Immunohistochemical analysis of p501 protein expression using a rabbit polyclonal antibody raised against the human p501 protein (4.4 µg ml–1) in a prostate sample from a control monkey (A), pancreas sample from a monkey injected with p501 + AS15 (B), on vessel walls of the brain cortex sample from a monkey injected with AS15 immunostimulant (D), and in stomach pit cells and glands from a monkey injected with p501 + AS15 (F) (study 4; p501 + AS15). Immunostaining specificity was demonstrated by lack of immunopositive response using anti‐OspA antibody (2.5 µg ml–1) (C,E,G).
Discussion
The non‐clinical repeated‐dose toxicity studies presented herein evaluated potential local and systemic toxic effects of several cancer vaccine candidates, containing the AS15 immunostimulant and a target tumor antigen (WT1, p501, recPRAME or dHER2) in animal models.
A summary of the main findings from all studies is shown in Table 5. In rabbits, no significant systemic toxicity after seven repeated injections of WT1 + AS15 was observed. The post‐injection changes in several hematology and biochemistry parameters were mostly transient, related to local inflammation, and within the pre‐dose and (historical) control ranges. Locally, injections of AS15 and WT1 + AS15 induced mononuclear or mixed cell infiltrates in the injected muscles. In addition, mineralization and necrosis, associated with the inflammation, were observed in the WT1 + AS15 groups, but not with AS15 alone.
Table 5.
Summary of main findings (all studies)
| Study | Candidate vaccine | Clinical signs (systemic/local) | Body weight | FC | Body temp | Blood H/B | Organ weights | Macroscopic examination | Microscopic examination of injection sites (histopathology) |
|---|---|---|---|---|---|---|---|---|---|
| Study in rabbits | |||||||||
| WT1 + AS15 | / | / | / | ↑ (WT1 + AS15; post 7) | H: ↑ F (tr gr; post 1 and 7) | / | Activated popliteal lymph nodes, lymphadenitis (WT1 + AS15) | Mononuclear cell infiltrate, necrosis, mineralization (tr gr; post 7) | |
| Inj site hematoma (all gr) | B: TP; ↓ A, A/G (tr gr; post 1 and 7) | ||||||||
| Studies in monkeys | |||||||||
| 1 | p501 + AS15 (7 inj) | / | ↓ (tr gr) | / | / | H: ↑ WBC (tr gr; post 1); N, M (tr gr, post 7) | / | / | Myodegeneration, necrosis, sarcolemmal nuclei proliferation, interstitial inflammatory cells, interstitial fibrosis (tr gr; last inj site) |
| / | B: ↓ A/G (tr gr; post 1, and 7) | ||||||||
| 2 | recPRAME + AS15 (7 inj) | Erythema and edema (tr gr) | / | / | ↑ (tr gr; post 1) | H: ↑ N, F, APTT (tr gr; post 1 and 7) | ↑ axillary lymph nodes (tr gr; post tr period) | Enlarged iliac, axillary and popliteal lymph nodes (1 male tr gr); enlarged axillary lymph nodes (1 female tr gr) | Mononuclear cell infiltrate, interstitial hemorrhages, fibroplasia/fibrosis (tr gr; last inj site) |
| B: / | ↑ iliac, axillary, popliteal, and inguinal lymph nodes (tr gr; post tr‐free period) | ||||||||
| 3 | dHER2 + AS15 (20 inj) | Erythema and edema (tr gr) | / | / | ↑ (tr gr; 4/24h post 1, 2, 4) | H: ↑ F (tr gr; post 2, 3, 4); ↓ RBC (post 1); PCV (post 1,2); Hb (post 1, 2, 4); tr gr | / | / | Macrophage infiltrates (aponeurosis, tr gr; last inj site) |
| Myodegeneration/necrosis (all gr) | |||||||||
| B: ↑ Bil/Urea/Creat (tr gr; post 1, 2, 3, 4) | |||||||||
| 4 | p501 + AS15 (20 inj) | / | ↓ (tr gr) | / | / | H: ↑ F (tr gr; post 1, 3, 4); ↑ WBC | ↑ prostate (p501 + AS15) | Epithelial prostate cell hypertrophy (p501 + AS15) | Hemorrhage, perivascular neutrophilic infiltration, interstitial edema and interstitial fibrosis (all gr) |
| / | (p501 + AS15; post 1, 4); ↓ L (tr gr; post 2, 3, 4) | ↓ prostate (AS15) | |||||||
| Multifocal slight segmental chronic periarteritis in both kidneys (1 monkey, p501 + AS15) | |||||||||
| ↓ thymus and spleen (p501 + AS15) | Hematomas of inj sites (control, AS15) | ||||||||
| B: / | ↓ thyroid (tr gr) | ||||||||
, no change in parameter; A, albumin; APTT, Activated partial thromboplastin time; B, blood biochemistry parameters; Bil, bilirubin; Creat, creatinine; F, fibrinogen; FC, food consumption; G, globulin; gr, group; H, blood hematology parameters; Hb, hemoglobin; inj, injection; M, monocytes; N, neutrophils; PCV, packed cell volume (hematocrit); RBC, red blood cells; temp, temperature; TP, total protein; tr, treatment; WBC, white blood cells.
Injections of saline also induced slight inflammation of mononuclear cell type, which is considered spontaneous or background pathology (very slight) or a normal response to the injection procedure (Thuilliez et al., 2009). In monkeys, seven or 20 repeated injections of p501 + AS15, recPRAME + AS15 or dHER2 + AS15 were well tolerated.
There was no observed change in the volume of the prostate for monkeys injected with AS15 or p501 + AS15 while a volume increase was observed in controls (study 4). At necropsy, prostate weights were higher in the vaccine group (and correlated with epithelial cell hypertrophy) and lower in the AS15 group, in comparison to controls. Organ weights for male reproductive organs such as prostate, seminal vesicles or testes usually correlate with age and weight but can be also influenced by other factors, such as sexual maturity of the individual, prostatic hypertrophy or other pathological conditions, social ranking or state of repletion (Chapin and Creasy, 2012; Haruyama et al., 2012; Ku et al., 2010). In this study, terminal prostate volume correlated well with terminal body weight. Thus, it is likely that the observed differences between the control and the treated monkeys resulted from differences in weight/size of the animals or their sexual maturity and not from vaccination with AS15 or p501 + AS15. Epithelial hypertrophy in the prostate was no longer present at the end of the treatment‐free period. There was no other evidence of systemic toxicity. No other signs of systemic toxicity were observed in any of the studies.
Following seven injections of p501 + AS15 or recPRAME + AS15, or 20 injections of dHER2 + AS15 or AS15, only local injection site reactions, limited to edema and erythema at the injection sites, were observed. Following injections of recPRAME + AS15, this correlated with interstitial inflammatory cell infiltrate and edema, as well as occasional interstitial hemorrhages and fibroplasia/fibrosis. In addition, after seven injections of p501 + AS15, body weight gain was slightly lower than that of monkeys injected with saline. Another observed effect was higher post‐injection body temperature after seven injections of recPRAME + AS15 or 20 injections of dHER2 + AS15 compared with the control groups.
Transient elevations of a few hematology (WBC [neutrophil and eosinophil], fibrinogen, APTT) or biochemistry variables (bilirubin, urea, creatinine) were observed in all studies in monkeys injected with the candidate vaccines or AS15; all parameters returned to physiological values before the next injection or at the end of the recovery period. Various immunostimulants have been shown to act locally by specifically stimulating the innate immune system at the site of injection and draining lymph nodes (Didierlaurent et al., 2009, 2014; Morel et al., 2011). This activation is transient and helps to stimulate antigen‐specific immune responses. Local activation can lead to systemic effect via spillover of inflammatory mediators or egress of progenitors from bone marrow but this cannot be addressed in our studies.
Combination of different immunostimulants is thought to improve vaccine efficacy. AS15 is a mixture of immunostimulants, i.e. MPL, QS‐21 and CpG; to date, these components have been tested alone or in combination with other immunostimulants in various vaccines that are either licensed or in development. MPL is a component of the AS04 Adjuvant System used in the human papillomavirus‐16/18 AS04‐adjuvanted vaccine (Cervarix®; GSK, Rixensart, Belgium). A previous study assessing the contribution of MPL in response to AS04, demonstrated a localized and transient effect of MPL after intramuscular immunization with no evidence of systemic effects (Didierlaurent et al., 2009). Transient activation of an innate response at the injection site was also observed after administration of the AS01 Adjuvant System, containing MPL and QS‐21 (Didierlaurent et al., 2014). Furthermore, no signs of systemic toxicity were observed after single or repeated administrations of the malaria candidate vaccines formulated with AS01 or AS02 Adjuvant Systems in rabbits (Segal et al., 2015). CpG ODNs have been tested as adjuvants in a number of non‐clinical and phase I–III clinical trials testing vaccines against infectious diseases (Cooper et al., 2004a,2004b; Klinman et al., 2006; Mullen et al., 2008) and cancer (Brody et al., 2010; Pilon‐Thomas et al., 2006; Speiser et al., 2005; Valmori et al., 2007). In previous studies in non‐human primates or in humans, no significant toxicities were reported after injections of CpG 7909 even at high doses (Krieg et al., 2004; Stewart et al., 2008). In two phase I clinical trials in healthy volunteers, injections of CpG 7909 were well tolerated and only transient injection site reactions and flu‐like symptoms were observed with no evidence of organ toxicity (Krieg et al., 2004). In addition, several previous studies assessed the effects of the combination of CpG with other immunostimulants. Intramuscular injections of mice with CpG combined with MPL caused only mild damage to the injection site muscles (Weeratna et al., 2000). In a phase I clinical trial in patients with advanced melanoma, CpG ODNs enhanced the adjuvant effect of incomplete Freund's adjuvant, resulting in significantly higher CD8+ T‐cell responses as compared to vaccination without CpG ODNs (Speiser et al., 2005). In this previous study, no major systemic effects were observed although all patients developed inflammatory signs at the injection sites. Altogether, these and our data suggest that vaccine formulations combining MPL, QS‐21 and CpG (AS15) are well tolerated, supporting the use of AS15 as a vaccine adjuvant in future clinical research.
Following seven injections of p501 + AS15 or recPRAME + AS15, or 20 injections of dHER2 + AS15 or AS15, microscopic examinations of the injection sites at the end of the treatment periods revealed histopathological changes, including: myodegeneration/necrosis, sacrolemmal nuclei proliferation, interstitial inflammatory cells and interstitial fibrosis (study 1); interstitial inflammatory cell infiltrate and edema, occasional interstitial hemorrhages and fibroplasia/fibrosis, increased weights of the axillary lymph nodes, which drained the last injected site (study 2); and transient inflammatory cell infiltration at the injection site of the last injected muscle (study 3).
In all studies, injections of the candidate vaccines induced robust antibody responses (p501‐specific, PRAME‐specific or dHER2‐specific). Based on these findings, and because of the similar expression pattern of the p501 protein in the prostate of adult monkeys and humans (evaluated in studies 1 and 4) or a high sequence homology between the monkey and human PRAME in terms of the coding nucleotide and protein (>90%) sequence, we concluded that the cynomolgus monkey was a suitable immunological responder species for these candidate vaccines.
All results presented in this manuscript were consistent with a previous manuscript disclosing the potential local and systemic toxicities induced by single and up to 25 repeated injections of the MAGE‐A3 antigen combined with AS15 in rabbits and cynomolgus monkeys, respectively (Destexhe et al., 2014a). In this previous study, injection(s) were systemically well tolerated with only local reactions observed at the injection sites after repeated administrations in monkeys. Antigen‐specific antibody and CD4+ T‐cell responses were detectable up to 3 months after the last injection in monkeys. Furthermore, we have also previously assessed the effects of AS15 alone or combined with MAGE‐A3 on fertility and pre‐ and postnatal development in rats and monkeys. Similarly, no signs of systemic toxicity were observed after administration of AS15 or MAGE‐A3 + AS15 in these animals (Destexhe et al., 2014b).
The purpose of the toxicity studies reported in this manuscript was to assess the potential test item‐ and immune‐related toxicities associated with repeated injections of the different cancer vaccine candidates. The route of administration, the dose of the injected test item and the administration schedule were influenced by the future clinical trial designs. The designs of the toxicity studies were also chosen to ensure an overexposure of the animals to the test items (based on a comparison of animal to human body weight) as well as a higher frequency of exposure than in the future clinical setting, and that an optimal immune response has been induced in all animals. Furthermore, as the potential cancer vaccines tested in our studies are thought to induce immune (antibody and T‐cell) responses against WT1, p501, dHER2 or PRAME cancer antigens that are also self‐antigens for humans, it was important to verify the absence of signs of inflammation or cytotoxicity potentially induced by these vaccines in normal tissues. Overall, the results of these non‐clinical studies suggest that repeated intramuscular injections of the AS15 immunostimulant combined with the tumor antigens p501, recPRAME, WT1 or dHER2 were systemically well tolerated by rabbits and cynomolgus monkeys under the experimental conditions used, further supporting the choice of AS15 for the use in immunotherapeutic cancer vaccines.
Conclusions
Repeated injections (up to 20) of a full human dose of the candidate cancer vaccines containing the AS15 immunostimulant and various tumor antigens were systemically well tolerated by rabbits and cynomolgus monkeys, with mostly local reactions observed at the injection sites. All potential target tumor antigens combined with the AS15 immunostimulant‐induced vigorous antibody responses in rabbits or monkeys accompanied by mild inflammatory responses. The findings from these non‐clinical studies suggest a favorable safety profile of the AS15 immunostimulant and support its use for clinical development of potential immunotherapeutic cancer vaccines.
Funding
GlaxoSmithKline Biologicals SA was the funding source of the study conduct and analysis and they funded all costs associated with the development and the publishing of the current paper.
Authors contributions
All authors except L.S. and F.K. were involved in study design. All authors were involved in data analyses and interpretation. J.S. was study director at CiToxLAB France. N.B. and C.G. were involved in serology testing at GSK Vaccines. F.K. was involved in histopathology analyses performed at TNO Quality of Life. All authors were involved in drafting and approval of the manuscript.
Conflict of interest
N.B., C.G. and L.S. are employees of GSK Vaccines (N.G. was an employee of GSK Vaccines at the time of the study). C.G. and N.G. declare stock ownership in the GSK group of companies. C.G. and N.G. are also inventor on patents owned by GSK group of companies. J.S. and R.F. are employees of CiToxLAB France; F.K. is employee of TNO Quality of Life; two CROs (Contract Research Organizations) where the safety studies have been performed on a contractual basis with GlaxoSmithKline Biologicals SA.
Supporting information
Supporting info item
Acknowledgments
We are grateful to Dr. M.K. Prinsen (TNO Quality of Life) and to teams in GSK Vaccines for their contribution to these studies and in particular to S. Veenstra for studies coordination and M. Bisteau for IHC analyses. We also thank Urszula Miecielica, PhD, Mihai Surducan, PhD (XPE Pharma & Science) and Virginie Durbecq, PhD (XPE Pharma & Science, on behalf of GSK Vaccines) for providing medical writing services and editorial support in preparing this manuscript.
Garçon, N. , Silvano, J. , Kuper, C. F. , Baudson, N. , Gérard, C. , Forster, R. , and Segal, L. (2016) Non‐clinical safety evaluation of repeated intramuscular administration of the AS15 immunostimulant combined with various antigens in rabbits and cynomolgus monkeys. J. Appl. Toxicol., 36: 238–256. doi: 10.1002/jat.3167.
References
- Aldrich JF, Lowe DB, Shearer MH, Winn RE, Jumper CA, Kennedy RC. 2010. Vaccines and immunotherapeutics for the treatment of malignant disease. Clin. Dev. Immunol. 2010: 697158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic M, Gulley JL. 2012. Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol. Immunother. 61: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R. 2010. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 28: 4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al 1990. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60: 509–520. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Creasy DM. 2012. Assessment of circulating hormones in regulatory toxicity studies II. Male reproductive hormones. Toxicol. Pathol. 40: 1063–1078. [DOI] [PubMed] [Google Scholar]
- Cluff CW. 2010. Monophosphoryl lipid A (MPL) as an adjuvant for anti‐cancer vaccines: clinical results. Adv. Exp. Med. Biol. 667: 111–123. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. 2004a. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix‐B HBV vaccine in healthy adults: a double‐blind phase I/II study. J. Clin. Immunol. 24: 693–701. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, Laframboise C, Al Adhami MJ, Khaliq Y, Seguin I, Cameron DW. 2004b. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 22: 3136–3143. [DOI] [PubMed] [Google Scholar]
- Destexhe E, Grosdidier E, Baudson N, Forster R, Gerard C, Garcon N, Segal L. 2014a. Non‐clinical safety evaluation of single and repeated intramuscular administrations of MAGE‐A3 Cancer Immunotherapeutic in rabbits and cynomolgus monkeys. J. Appl. Toxicol. Sep 12. DOI: 10.1002/jat.3025 [DOI] [PubMed] [Google Scholar]
- Destexhe E, Stannard D, Wilby OK, Grosdidier E, Baudson N, Forster R, Gérard CM, Garçon N, Segal L. 2014b. Nonclinical reproductive and developmental safety evaluation of the MAGE‐A3 Cancer Immunotherapeutic, a therapeutic vaccine for cancer treatment. Reprod. Toxicol. 51C: 90–105. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garçon N. 2009. AS04, an aluminum salt‐ and TLR4 agonist‐based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183: 6186–6197. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, Dendouga N, Langlet C, Malissen B, Lambrecht BN, Garçon N, Van Mechelen M, Morel S. 2014. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 193: 1920–1930. [DOI] [PubMed] [Google Scholar]
- Dimberu PM, Leonhardt RM. 2011. Cancer immunotherapy takes a multi‐faceted approach to kick the immune system into gear. Yale J. Biol. Med. 84: 371–380. [PMC free article] [PubMed] [Google Scholar]
- European Agency for the Evaluation of Medicinal Products (EMA) . Committee for Proprietary Medicinal Products (CPMP) . 1997. Note for guidance on preclinical pharmacological and toxicological testing of vaccines. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004004.pdf (accessed 27 February 2014).
- European Agency for the Evaluation of Medicinal Products (EMA) . Committee for Proprietary Medicinal Products (CPMP) . 1999. ICH Topic S 4 Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing). Note for Guidance on Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing (CPMP/ICH/300/95). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002800.pdf (accessed 27 February 2014).
- European Agency for the Evaluation of Medicinal Products (EMA) . Committee for proprietary Medicinal Products (CPMP) . 2000. Note for guidance on repeated dose toxicity. CPMP/SWP/1042/99. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003102.pdf (accessed 27 February 2014).
- European Agency for the Evaluation of Medicinal Products (EMA) . 2001. Note for guidance on non‐clinical local tolerance testing of medicinal products (CPMP/SWP/2145/00). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003315.pdf (accessed 27 February 2014).
- European Agency for the Evaluation of Medicinal Products (EMA) . Committee for proprietary Medicinal Products (CPMP) . 2005. Guideline on adjuvants in vaccines for human use. 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003809.pdf (accessed 13 February 2015).
- Epping MT, Bernards R. 2006. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 66: 10639–10642. [DOI] [PubMed] [Google Scholar]
- Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. 2005. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 122: 835–847. [DOI] [PubMed] [Google Scholar]
- Forster R. 2012. Study designs for the nonclinical safety testing of new vaccine products. J. Pharmacol. Toxicol. Methods 66: 1–7. [DOI] [PubMed] [Google Scholar]
- Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA. 1990. Homozygous deletion in Wilms tumours of a zinc‐finger gene identified by chromosome jumping. Nature 343: 774–778. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Rivoltini L, Levchenko E, Testori A, Utikal J, Ascierto P, Salaun B, Vanhoutte N, Gillet M, Brichard V. 2012. Immunogenicity and safety of the PRAME Immunotherapeutic in metastatic melanoma: A phase I/II dose escalation study. Ann. Oncol. 23(Suppl. 9): 1110. [Google Scholar]
- Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, Chen W, Castro H, Lehmann F, Spector N, Wei J, Osada T, Lyerly HK, Morse MA. 2012. Phase 1 clinical trial of HER2‐specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J. Transl. Med. 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama E, Suda M, Ayukawa Y, Kamura K, Mizutamari M, Ooshima Y, Tanimoto A. 2012. Testicular development in cynomolgus monkeys. Toxicol. Pathol. 40: 935–942. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Xie H, Ivins BE. 2006. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann. N. Y. Acad. Sci. 1082: 137–150. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. 2004. Induction of systemic TH1‐like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B‐class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27: 460–471. [DOI] [PubMed] [Google Scholar]
- Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion‐Sileni V, Maio M, Testori A, Dorval T, Grob JJ, Becker JC, Spatz A, Eggermont AM, Louahed J, Lehmann FF, Brichard VG, Keilholz U. 2013. Selection of immunostimulant AS15 for active immunization with MAGE‐A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 31: 2413–2420. [DOI] [PubMed] [Google Scholar]
- Ku WW, Pagliusi F, Foley G, Roesler A, Zimmerman T. 2010. A simple orchidometric method for the preliminary assessment of maturity status in male cynomolgus monkeys (Macaca fascicularis) used for nonclinical safety studies. J. Pharmacol. Toxicol. Methods 61: 32–37. [DOI] [PubMed] [Google Scholar]
- Lane Z, Hansel DE, Epstein JI. 2008. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. Am. J. Surg. Pathol. 32: 1322–1326. [DOI] [PubMed] [Google Scholar]
- Lee SB, Haber DA. 2001. Wilms tumor and the WT1 gene. Exp. Cell Res. 264: 74–99. [DOI] [PubMed] [Google Scholar]
- Ministère de l'Emploi et de la Solidarité . 2000. ARRÊTÉ DU 14 MARS 2000 relatif aux bonnes pratiques de laboratoire. http://ansm.sante.fr/var/ansm_site/storage/original/application/dd4641e3edd1885e60f0337d4c385b5e.pdf (accessed 27 February 2014).
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, Kielland A, Chomez P, Garçon N, Van Mechelen M. 2011. Adjuvant System AS03 containing alpha‐tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29: 2461–2473. [DOI] [PubMed] [Google Scholar]
- Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, Moretz S, Zhou H, Long CA, Miller LH, Treanor J. 2008. Phase 1 trial of AMA1‐C1/Alhydrogel plus CPG 7909: an asexual blood‐stage vaccine for Plasmodium falciparum malaria. PLoS One 3: e2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasomyon T, Samphao S, Sangkhathat S, Mahattanobon S, Graidist P. 2014. Correlation of Wilms' tumor 1 isoforms with HER2 and ER‐alpha and its oncogenic role in breast cancer. Anticancer Res 34: 1333–1342. [PubMed] [Google Scholar]
- OECD . 1998. Organisation for Economic Co‐operation and Development. OECD SERIES ON PRINCIPLES OF GOOD LABORATORY PRACTICE AND COMPLIANCE MONITORING. OECD Principles on Good Laboratory Practice (as revised in 1997) (ENV/MC/CHEM(98)17).
- Oka Y, Tsuboi A, Elisseeva OA, Udaka K, Sugiyama H. 2002. WT1 as a novel target antigen for cancer immunotherapy. Curr. Cancer Drug Targets 2: 45–54. [DOI] [PubMed] [Google Scholar]
- Pilon‐Thomas S, Li W, Briggs JJ, Djeu J, Mule JJ, Riker AI. 2006. Immunostimulatory effects of CpG‐ODN upon dendritic cell‐based immunotherapy in a murine melanoma model. J. Immunother. 29: 381–387. [DOI] [PubMed] [Google Scholar]
- Pujol J‐L, De Pas T, Rittmeyer A, Kubisa B, Vallieres E, Levchenko E, Salaun B, Vanhoutte N, Debois M, Brichard V. 2012. Immunogenicity and safety of the PRAME Cancer Immunotherapeutic in non‐small cell lung cancer (NSCLC): A phase I dose escalation study. Ann. Oncol. 23(Suppl. 9): ix386 (abs1182P). [Google Scholar]
- Segal L, Morelle D, Blee M, Moore E, Damsten M, Liu KC, Destexhe E, Garçon N. 2015. Local tolerance and systemic toxicity of single and repeated intramuscular administrations of two different formulations of the RTS,S malaria candidate vaccine in rabbits. Regul. Toxicol. Pharmacol. 71: 269–278. [DOI] [PubMed] [Google Scholar]
- Sheridan T, Herawi M, Epstein JI, Illei PB. 2007. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am. J. Surg. Pathol. 31: 1351–1355. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 235: 177–182. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Lienard D, Rufer N, Rubio‐Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. 2005. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 115: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart VA, McGrath S, Krieg AM, Larson NS, Angov E, Smith CL, Brewer TG, Heppner DG Jr. 2008. Activation of innate immunity in healthy Macaca mulatta macaques by a single subcutaneous dose of GMP CpG 7909: safety data and interferon‐inducible protein‐10 kinetics for humans and macaques. Clin. Vaccine Immunol. 15: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuilliez C, Dorso L, Howroyd P, Gould S, Chanut F, Burnett R. 2009. Histopathological lesions following intramuscular administration of saline in laboratory rodents and rabbits. Exp. Toxicol. Pathol. 61: 13–21. [DOI] [PubMed] [Google Scholar]
- The European Parliament and the Council of the European Union . 2004. DIRECTIVE 2004/10/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 11 February 2004. Official Journal of the European Union L 50/59. http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:050:0028:0028:EN:PDF (accessed 27 February 2014).
- The Commission of the European Communities . 1999. Commission Directive 1999/11/EC of 8 March 1999. Official Journal of the European Communities L 77. http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1999:077:0008:0021:EN:PDF (accepted 27 February 2014).
- The Council of the European Communities . 1986. Council Directive of 24 November 1986. http://ec.europa.eu/food/fs/aw/aw_legislation/scientific/86‐609‐eec_en.pdf (accessed 27 February 2014).
- Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. 2007. Vaccination with NY‐ESO‐1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross‐priming. Proc. Natl. Acad. Sci. U. S. A. 104: 8947–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeratna RD, McCluskie MJ, Xu Y, Davis HL. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18: 1755–1762. [DOI] [PubMed] [Google Scholar]
- Weir GM, Liwski RS, Mansour M. 2011. Immune modulation by chemotherapy or immunotherapy to enhance cancer vaccines. Cancers (Basel) 3: 3114–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) . 2003. WHO guidelines on nonclinical evaluation of vaccines. http://www.who.int/biologicals/publications/nonclinical_evaluation_vaccines_nov_2003.pdf (accessed 27 February 2014).
- Xu C, Wu C, Xia Y, Zhong Z, Liu X, Xu J, Cui F, Chen B, Roe OD, Li A, Chen Y. 2013. WT1 promotes cell proliferation in non‐small cell lung cancer cell lines through up‐regulating cyclin D1 and p‐pRb in vitro and in vivo. PLoS One 8: e68837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


