Figure 1.
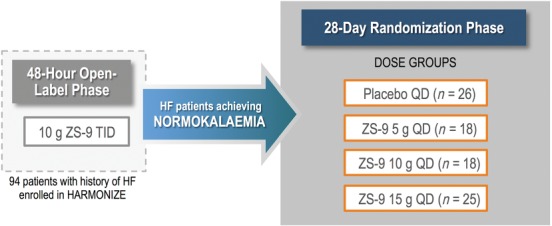
Study design overview. Patients with hyperkalaemia and a history of heart failure (HF) in the Hyperkalaemia Randomized Intervention Multidose ZS‐9 Maintenance (HARMONIZE) study received ZS‐9 10 g during the 48‐h open‐label phase. Those who achieved normokalaemia were randomized to placebo (randomized withdrawal) or ZS‐9 (5 g, 10 g, or 15 g) for 28 days.
