Abstract
Background
Standard of care for high-grade gliomas (HGGs) includes surgical debulking and adjuvant chemotherapy and radiation. Patients under treatment require frequent clinical and imaging monitoring for therapy modulation. Differentiating tumor progression from treatment-related changes can be challenging on conventional MRI, resulting in delayed recognition of tumor progression. Arterial spin labeling (ASL) may be more sensitive for tumor progression.
Materials and methods
ASL and associated conventional MR images obtained in patients previously treated for HGGs and before biopsy or re-resection were reviewed by three neuroradiologists who were blinded to the histopathologic results. Regions of interest (ROIs) of greatest perfusion were chosen by consensus, and mirror-image contralateral ROIs were also placed. Sensitivity of ASL for tumor progression was compared with those of contrast-enhanced T1-weighted (T1W-CE) and fluid-attenuated inversion recovery (FLAIR) images using McNemar’s test. We tested for an association between cerebral blood flow (CBF) and apparent diffusion correlation (ADC) using a Hotelling–Lawley trace. Finally, we used a Pearson’s analysis to test for a correlation between CBF and percentage of tumor within each resection.
Results
Twenty-two patients were studied. ASL demonstrated hyperperfusion in all cases with mean CBF ratio 3.37 (±1.71). T1W-CE and FLAIR images were positive in 15 (p = 0.0233) and 16 (p = 0.0412) cases, respectively. There was no association between ADC and CBF (p = 0.687). There was a correlation between CBF and percentage of tumor (p = 0.048).
Conclusion
ASL may be more sensitive than conventional MR sequences for the detection of tumor progression in patients treated for HGGs.
Keywords: Arterial spin labeling, MR perfusion, glioblastoma, radiation necrosis, pseudoprogression
Introduction
High-grade gliomas (HGGs) account for approximately 81% of malignant brain tumors in adults and carry a devastating prognosis.1,2 This disease can affect patients of any age and results in more lost years of life than any other brain tumor. Standard of care includes surgical debulking when possible as well as adjuvant chemotherapy and radiation therapy. Patients are closely monitored with magnetic resonance imaging (MRI) in order to detect the earliest signs of disease progression, which may prompt initiation of second- and third-line therapies while the performance status of patients allows additional treatment. However, conventional MRI often falls short of confidently distinguishing early stages of tumor progression from treatment-related changes, including necrosis and pseudoprogression. Both tumor progression and treatment-related changes can exhibit hyperintense signal on fluid-attenuated inversion recovery (FLAIR) images, mass effect and contrast enhancement.3,4 Therefore more advanced imaging methods are needed to reliably differentiate these processes in order to optimize therapy modulation.
MR perfusion may offer one such method to differentiate the more metabolically active and hyperemic tumor progression from the relatively ischemic treatment-related changes. The first iterations of MR perfusion technology, including dynamic susceptibility contrast (DSC) and dynamic contrast enhancement (DCE), require an intravenous (IV) bolus of gadolinium-based contrast and provide a map from which relative changes in perfusion can be detected.
The newer arterial spin labeling (ASL) MR perfusion imaging offers a quantitative alternative to contrast-enhanced perfusion imaging, and does not require gadolinium-based contrast. Previously published data supporting the use of ASL for evaluation of progression from treatment-related changes are limited by both qualitative approaches, and, more important, the lack of pathological confirmation of disease status, with reliance on clinical and/or radiographic stability over time as evidence of absence of disease progression.5,6
The purpose of this study is to quantitatively describe ASL perfusion characteristics using the largest series of biopsy-proven tumor progression. We compared the effectiveness of ASL with conventional contrast-enhanced T1-weighted (T1W-CE), T2-weighted FLAIR and apparent diffusion correlation (ADC) maps for the detection of tumor progression. We then correlated the absolute value of cerebral blood flow (CBF) with the ratio of tumor vs treatment-related changes within the sample. We hypothesized that ASL would be more sensitive than T1W-CE or FLAIR for detection of tumor progression and that ADC values would be lower within the tumor compared with the contralateral side. We also hypothesized that CBF would directly correlate with the degree of tumor burden in the histologic samples.
Materials and methods
The study was institutional review board approved and Health Insurance Portability and Accountability Act (HIPAA) compliant. A retrospective review of pathology reports from patients with suspected recurrent or progressive HGG (World Health Organization Grade III or IV) including anaplastic astrocytoma and glioblastoma diagnosed at University of Colorado Hospital from 2011 to 2014 was performed. Patients were included if they had previously been treated with chemotherapy, radiation and surgery according to standard of care, and later underwent biopsy or re-resection during follow-up for suspected disease progression, and had diagnostic ASL imaging performed on a single 3 T MRI scanner prior to biopsy. None of the patients in this study had been treated with anti-angiogenic agents.
Imaging protocol
All patients were imaged on a Signa HDxt 3.0 T MRI scanner (GE Healthcare, Milwaukee, WI). MRI examination protocols included T1W fast spin echo (FSE) imaging before and after weight-based IV administration of gadobenate dimeglumine (section thickness, 5 mm; gap 0.5 mm), T2W (section thickness, 5 mm; gap 0.5 mm), T2-FLAIR (inversion time (TI) = 2800; section thickness, 5 mm; gap 0.5 mm), diffusion-weighted (three directions, b = 1000 s/mm2; section thickness, 5 mm; gap 0.5 mm) and GE three-dimensional (3D) pseudo-continuous ASL imaging (points, 512; arms, 8; nex, 3.00; bandwidth, 62.5; post-label delay, 2025.0 ms; section thickness, 4 mm; gap 0 mm). Crusher gradients were applied to ASL source data eliminating potential arterial signal. Based on the ASL data set, CBF maps were generated using the GE FuncTool software suite (version HDxp 23.0).
Imaging assessment
Three subspecialty certified neuroradiologists (“reviewers”) reviewed pre-biopsy ASL images for all participants. Although all biopsies ultimately proved tumor progression, reviewers were blinded to this at the time of evaluation. Using the post-biopsy T1W-CE images in conjunction with the operative note as a reference, the biopsy site was delineated on the pre-biopsy T1W-CE images and subsequently on the corresponding ASL images using a localizer. A uniformly sized region of interest (ROI) measuring 0.44 cm2 was then placed on the ASL image in the region of highest signal within the biopsy site. An additional ROI was then placed at the mirror-image site on the contralateral side, with attention paid to match tissue type (i.e. gray and/or white matter) as a proxy for normal perfusion. ROIs were selected by consensus by the reviewers. Reviewers were blinded to the other prior, concurrent and subsequent diagnostic conventional MRI sequences and to the radiology reports. ASL was deemed positive for tumor progression if lesional CBF was increased relative to the contralateral side. CBF ratio as defined by CBFlesional/CBFcontralateral was calculated. Lesional and contralateral ADC values were also recorded.
Following a one-month hiatus to prevent recall bias, the T1W-CE and FLAIR images from studies concurrent with and prior to the ASL images were reviewed for signs of disease progression. T1W-CE and FLAIR images were assessed in accordance with the Response Assessment in Neuro-Oncology (RANO) response criteria.
Finally, post hoc review of the patient database was performed to consider any previous available ASL and conventional imaging, and descriptive statistics were calculated.
Histopathological assessment
A single neuropathologist (BKD) with more than 20 years’ experience, blinded to radiologic findings, reviewed each slide to estimate the percentage of tumor progression and treatment-related changes. Resected cortex or fibrotic areas from previous surgical procedures were not included in the denominator. Semi-quantitative scoring was performed visually. Samples that contained material other than tumor or treatment-related changes, e.g. one case with Surgicel granuloma, were excluded. All slides were reviewed without the original pathologic report, although post hoc comparison of the reports provided for this study with the original clinical reports revealed no substantial differences.
A second analysis was performed correlating the percentage of tumor in the sample with the degree of CBF. Five samples obtained from stereotactic biopsies were excluded from the second analysis, as they were not believed to be representative of the entire tumor. Gross samples from two other patients who demonstrated predominantly tumoral microcysts were also excluded from this comparison.
Statistical methods
Patient demographics were collected from the medical record. Clinical details including tumor grade and time interval between ASL imaging and biopsy were recorded. Descriptive statistics were calculated for patient demographics and clinical findings. Paired t-tests were used to compare mean CBF in the tumor with corresponding CBF values on the unaffected side. A general linear multivariate model7 was used to evaluate the association between ADC and CBF on the lesion side. Outcomes included intra-voxel CBF within the lesional ROI. The Hotelling–Lawley trace was used to test the null hypothesis of no association between ADC and lesional CBF, at an alpha level of 0.05, using ADC as the primary predictor. The sensitivity of ASL was compared with the sensitivities of T1W-CE and FLAIR sequences for identification of tumor progression using McNemar’s tests with continuity correction. Percentage of tumor was compared with degree of CBF using a Pearson’s correlation.
Results
Twenty-two patients were included. All biopsies included in this study were positive for disease progression and all resection samples demonstrated greater than 50% tumor (55–95%). The median time between ASL imaging and biopsy was 15.5 days (range: 2 to 489 days). Demographic and tumor characteristics are shown in Table 1.
Table 1.
Patient demographics and clinical features.
| Age | 50.7 ± 11.0 years |
| Gender | |
| Male | 11 (50%) |
| Female | 11 (50%) |
| Pre-biopsy diagnosis | |
| Low-grade glioma | 6 (27.3%) |
| High-grade glioma | 16 (72.7%) |
| Post-biopsy diagnosis | |
| Grade III | 8 (36.4%) |
| Grade IV | 14 (63.6%) |
| Mean CBF | |
| Tumor | 71.0 ± 34.7 ml/100 g/min |
| Contralateral | 24.0 ± 12.4 ml/100 g/min |
| Ratio | 3.37 ± 1.71 |
CBF: cerebral blood flow.
Sensitivity for tumor detection
ASL was positive in all cases. Tumoral CBF ranged from 22 to 175 ml/100 g/min, with a mean of 71.0 ± 34.7 ml/100g/min on the lesional side, and 24.0 ± 12.4 ml/100 g/min on the contralateral side (p < 0.001). Relative CBF ratio ranged from 1.65 to 7.62 with a mean of 3.37 ± 1.71.
T1W-CE and FLAIR images suggested tumor progression in 15 and 16 cases, respectively, yielding sensitivities of 68.2% and 72.7%, respectively. ASL was significantly more sensitive than both T1W-CE (p = 0.0233) and FLAIR (p = 0.0412) sequences. Mean ADC was 976.05 ± 335.96 × 10–6 mm2/sec; there was no association between ADC and CBF (p = 0.687).
Tumor burden and CBF
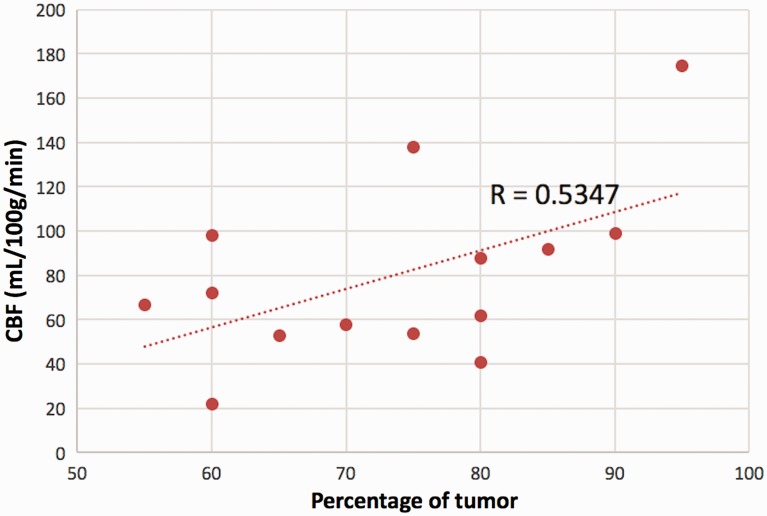
Fourteen pathological samples were deemed suitable for estimation of tumor percentage. Tumor percentage ranged from 55% to 95% with a mean of 73.6 ± 12.3%. Pearson’s analysis revealed a significant correlation between the proportion of tumor burden and degree of hyperperfusion r = 0.5347 (p = 0.048). This correlation is demonstrated in Figure 1.
Figure 1.
Cerebral blood flow (CBF) increases with degree of tumor burden within a sample (p = 0.048).
Review of available prior imaging
Post hoc review of the medical record revealed previous MRI for all but three of the patients in our series; one of these three was negative for both T1W-CE and FLAIR changes prior to biopsy. Thirteen patients with T1W-CE positivity on the pre-biopsy scan had at least one prior MRI with ASL, and five of these (38.5%) had ASL positivity prior to contrast enhancement. Fourteen patients with FLAIR positivity on the pre-biopsy study had one such study, and three of these (21.4%) demonstrated ASL positivity prior to FLAIR positivity. ASL was found to be positive up to 20.5 months prior to T1W-CE and up to 10.5 months prior to FLAIR. In none of the cases were T1W-CE or FLAIR images positive before ASL.
Discussion
Biology of tumor and treatment
Tumor progression and treatment response each involves a complex interplay of the proliferative changes of vasculogenesis and infiltration of viable tumor cells as well as multiple therapeutic effects including endothelial cell death, vascular thrombosis, hemorrhage and tumoral and neuronal cell death. These processes occur in the setting of a disrupted blood-brain barrier and increased interstitial water, and distinguishing these phenomena on conventional MRI has proved difficult.8–12
However, these processes differ markedly in their metabolic demand and required blood supply. Neovascularization is an early step in tumor growth and is required to facilitate the diffusion of oxygen and other metabolites if a tumor is to grow beyond the tissue of origin. HGGs can recruit nutrient supply by vascular cooption, angiogenesis, vasculogenesis, and transdifferentiation of relatively pleuripotent glioma cells into endothelial cells and pericytes.13 This neovasculature intermixes with native vasculature, facilitating hyperperfusion relative to normal brain. The importance of neovascularization in tumor characterization and grading has been well established,14–16 and it has been shown to be an independent prognostic indicator.13,17
This state of vascular proliferation is in sharp contrast to the relatively ischemic state found with treatment-related changes.18 Perfusion imaging highlights this important difference, and even a mild increase in perfusion may represent tumor progression (Figure 2). Moreover, this state of hyperperfusion precedes the more macroscopic tissue changes detectable by conventional images. DSC perfusion has been shown to demonstrate hyperperfusion up to a year before contrast enhancement develops.15,19 Likewise, in our series, of the patients with positive findings on conventional imaging on their pre-biopsy MRI, ASL was positive on one or more previous MRI studies prior to T1W-CE in 38.5% of cases and prior to FLAIR in 21.4% of cases (Figure 3).
Figure 2.
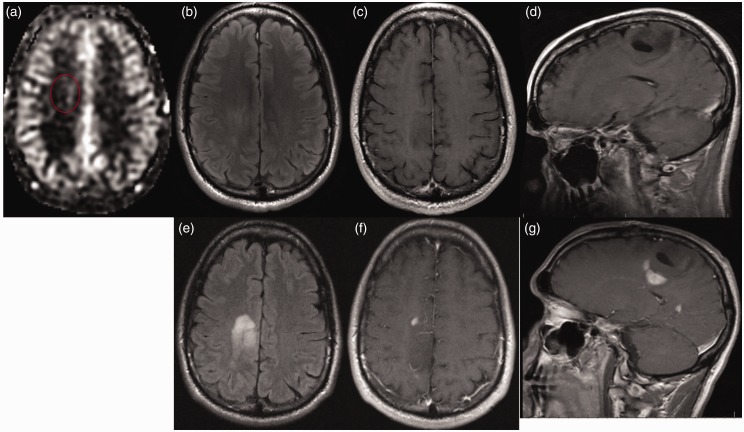
A 34-year-old man with history of treated WHO Grade II glioma nine years earlier presented with seizures. (a) ASL imaging demonstrates subtle hyperperfusion while (b) FLAIR and ((c) and (d)) T1W-CE images are unrevealing. Two months later tumor progression is readily evident on (e) FLAIR and ((f) and (g)) T1W-CE images (ASL images were not obtained at that time). Biopsy revealed hypercellular infiltrating WHO Grade IV glioblastoma positive for Ki-67 labeling, IDH1 mutation and p53 immunoreactivity. Even subtle changes on ASL, 34 ml/100 g/min in this case, may represent sentinel evidence of progression, particularly when the rCBF ratio is elevated, which was 1.9 in this case.
WHO: World Health Organization; ASL: arterial spin labeling; FLAIR: fluid-attenuated inversion recovery; T1W-CE: contrast-enhanced T1-weighted; IDH1: isocitrate dehydrogenase 1; rCBF: relative CBF.
Figure 3.
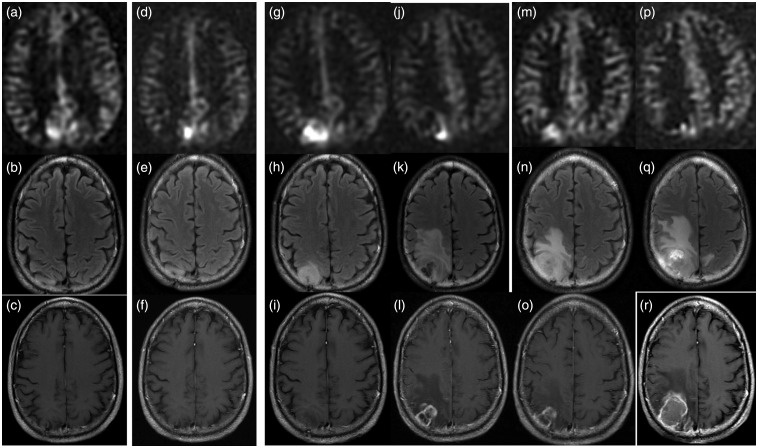
A 50-year-old male with treated WHO Grade II oligodendroglioma was treated with surgery, chemotherapy and radiation eight years previously. Surveillance scan demonstrates (a) ASL-positive nodule in the right precuneus, while the (b) FLAIR and (c) T1W-CE scans are unrevealing. The (h) FLAIR and (i) T1W-CE images became concerning seven months after the onset of hyperperfusion, and it was at this point adjuvant chemotherapy and radiation were re-initiated. ASL signal appears to scale roughly with the growth of solidly enhancing tumor, while the treatment-related change anterior to the tumor evident at 11 months demonstrates no hyperperfusion ((j)–(l)). By the time the patient underwent re-resection the lesion was quite necrotic and CBF was only 22 ml/100 g/min ((p)–(r)). Histopathology demonstrated WHO Grade IV glioblastoma with oligodendroglial components, densely cellular tumor with pseudopalisading necrosis and no treatment-related necrosis. Genetics demonstrated 1p and 19q deletions and IDH1 mutation.
WHO: World Health Organization; ASL: arterial spin labeling; FLAIR: fluid-attenuated inversion recovery; T1W-CE: contrast-enhanced T1-weighted; IDH1: isocitrate dehydrogenase 1.
ASL has been shown to correlate histopathologically with vascular proliferation, defined by the number of microvessels per sample.20 The clinical utility of this has been shown by previous investigators who demonstrated that contrast-enhanced MR perfusion imaging may have a significant impact on therapeutic decision making when interpreted and enacted on by experienced multidisciplinary neuro-oncological teams.21 Furthermore, quantification of tumoral CBF has been shown to demonstrate good to excellent inter-observer reproducibility.22
We performed a post hoc analysis to investigate whether there was a correlation between the percentage of tumor within the resection sample and the quantitative measure of CBF. Despite the rather small sample size for this sub-analysis with only 14 individuals, the correlation between these metrics was significant (p = 0.048). However, given the variability of perfusion we observed in absolute terms, heterogeneity of tumor genetics and subtypes, confounding effects of anti-angiogenetic drugs, etc., it would be premature to extrapolate these data to infer that absence of hyperperfusion implies absence of viable tumor.
It has been suggested that restricted diffusion on ADC images may suggest tumor progression.23–25 Our tumoral ADC values tended to fall within the threshold of less than 1300 × 10–6 mm2/sec described by Bulik et al.;25 however, this finding lacked specificity in our population and warrants further investigation.
ASL versus dynamic contrast methods
Dynamic contrast-bolused perfusion techniques were developed prior to ASL and are supported by a broader body of literature. ASL has been criticized as suffering from low signal to noise relative to contrast-bolused perfusion methods; however,26 more recent studies have shown that ASL may be preferred compared with DSC in hyperperfused tissue.27 Indeed, in this series, ASL signal was able to show excellent quantitative differentiation between tumor and normal tissue. DSC also assumes an intact blood-brain barrier. Various methods are typically applied including smoothing algorithms and preloading of contrast, to attempt to correct for this shortcoming. ASL is less prone to susceptibility artifacts than DSC.11 Without need of an exogenous contrast agent, ASL does not require specific bolus timing and is repeatable. ASL has the notable advantage of offering quantification of CBF, rather than a qualitative assessment.
Finally, these data are timely for another reason. Gadolinium-based contrast agents were assumed to be routinely chelated, excreted and eliminated. After years of widespread usage, that view began to change as the very rare phenomenon of nephrogenic systemic fibrosis became widely publicized. Until recently, lack of complete clearance was thought to be found only in patients with renal failure. Recently, however, T1 shortening has been described in the basal ganglia and dentate nuclei of patients who had received gadodiamide and gadopentatate dimeglumine.28–30 Autopsy reports have demonstrated that gadolinium can cross the blood-brain barrier and deposit within neuronal interstitium after as few as four lifetime doses, regardless of hepatorenal function.31 Potential clinical implications of this are yet unknown. Although macrocyclic gadolinium-based contrast agents may prove to be much safer and will likely play an important role in clinical imaging for some time, it is not unreasonable to begin considering post-gadolinium-era paradigms.
Limitations
The lack of samples with predominantly treatment-related changes or pseudoprogression in our data precluded an analysis of specificity of ASL in this population. This reflects the reluctance of neurosurgeons at our institution to operate without considerable suspicion for disease progression. Therefore, it is rare for biopsies to return findings other than tumor progression. The ability of ASL to differentiate between true response and pseudoresponse of patients receiving anti-angiogenic therapy such as bevacizumab on ASL perfusion imaging was also not evaluated because of lack of an adequate sample size.
Conclusion
We present the largest series of biopsy-proven progression of treated HGGs studied with ASL. ASL is a readily available MRI sequence that can reliably detect disease progression and was more sensitive to this change than conventional T1W-CE and FLAIR. The degree of CBF may correlate with tumor burden in the sampled tissue. In our cases of tumor progression in which T1W-CE and FLAIR as well as ASL sequences were positive, ASL detected tumor progression earlier than either of these conventional sequences in 21–38% of cases. ASL offers several advantages over the more widely used DSC including the lack of need for gadolinium-based contrast agents and quantitative cerebral perfusion maps.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2006; 2: 494–503. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol 2014; 16: 896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004; 63: 535–537. [DOI] [PubMed] [Google Scholar]
- 4.Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist 2003; 9: 180–188. [DOI] [PubMed] [Google Scholar]
- 5.Choi YJ, Kim HS, Jahng GH, et al. Pseudoprogression in patients with glioblastoma: Added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol 2013; 54: 448–454. [DOI] [PubMed] [Google Scholar]
- 6.Ozsunar Y, Mullins ME, Kwong K, et al. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol 2010; 17: 282–290. [DOI] [PubMed] [Google Scholar]
- 7.Muller KE, Stewart PW. Linear model theory: Univariate, multivariate, and mixed models, Hoboken, NJ: Wiley, 2006. [Google Scholar]
- 8.Cha J, Kim ST, Kim HJ, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol 2014; 35: 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma N, Cowperthwaite MC, Burnett MG, et al. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro Oncol 2013; 15: 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg 2010; 112: 758–765. [DOI] [PubMed] [Google Scholar]
- 11.Järnum H, Steffensen EG, Knutsson L, et al. Perfusion MRI of brain tumours: A comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010; 52: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins ME, Barest GD, Schaefer PW, et al. Radiation necrosis versus glioma recurrence: Conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005; 26: 1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 13.Dimberg A. The glioblastoma vasculature as a target for cancer therapy. Biochem Soc Trans 2014; 42: 1647–1652. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: Therapeutic implications. New Engl J Med 1971; 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 15.Kalpathy-Cramer J, Gerstner ER, Emblem KE, et al. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res 2014; 74: 4622–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson G, Mills SJ, Coope DJ, et al. Imaging biomarkers of angiogenesis and the microvascular environment in cerebral tumours. Br J Radiol 2011; 84(Spec No. 2): S127–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996; 77: 362–372. [DOI] [PubMed] [Google Scholar]
- 18.Tsien C, Galbán CJ, Chenevert TL, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol 2010; 28: 2293–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danchaivijitr N, Waldman AD, Tozer DJ, et al. Low-grade gliomas: Do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology 2008; 247: 170–178. [DOI] [PubMed] [Google Scholar]
- 20.Weber MA, Zoubaa S, Schlieter M, et al. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Cancer Imaging 2006; 6: S32–S41. [DOI] [PubMed] [Google Scholar]
- 21.Geer CP, Simonds J, Anvery A, et al. Does MR perfusion imaging impact management decisions for patients with brain tumors? A prospective study. AJNR Am J Neuroradiol 2012; 33: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai T, Kitajima M, Nakamura H, et al. Quantitative blood flow measurements in gliomas using arterial spin-labeling at 3T: Intermodality agreement and inter- and intraobserver reproducibility study. AJNR Am J Neuroradiol 2011; 32: 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deike K, Wiestler B, Graf M, et al. Prognostic value of combined visualization of MR diffusion and perfusion maps in glioblastoma. J Neurooncol 2016; 126: 463–472. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Lupo JM, Polley MY, et al. Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 2011; 13: 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulik M, Kazda T, Slampa P, et al. The diagnostic ability of follow-up imaging biomarkers after treatment of glioblastoma in the temozolomide era: Implications from proton MR spectroscopy and apparent diffusion coefficient mapping. Biomed Res Int 2015; 2015: 641023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatterpekar GM, Galheigo D, Narayana A, et al. Treatment-related change versus tumor recurrence in high-grade gliomas: A diagnostic conundrum—Use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 2012; 198: 19–26. [DOI] [PubMed] [Google Scholar]
- 27.Wang DJ, Alger JR, Qiao JX, et al. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: Comparison with dynamic susceptibility contrast-enhanced MRI. Stroke J Cereb Circ 2012; 43: 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 2016; 38: 331–336. [DOI] [PubMed] [Google Scholar]
- 29.Karabulut N. Gadolinium deposition in the brain: Another concern regarding gadolinium-based contrast agents. Diagn Interv Radiol 2015; 21: 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radbruch A, Weberling LD, Kieslich PJ, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: Evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol 2015; 50: 805–810. [DOI] [PubMed] [Google Scholar]
- 31.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275: 772–782. [DOI] [PubMed] [Google Scholar]