Abstract
Strawberry (Fragaria × ananassa) is an economically important soft fruit crop with polyploid genome which makes the breeding of new cultivars difficult. Simple and efficient method for transformation and regeneration is required for cultivars improvement in strawberry. In the present study, adventitious shoot regeneration has been investigated in three cultivated strawberry plants, i.e., Festival, Sweet Charly and Florida via direct organogenesis using the in vitro juvenile leaves as explants. Explants were collected after sub-culturing on a propagation medium composed of MS supplemented with 0.5 mg/l BA; 0.1 mg/l GA3 and 0.1 mg/l IBA. To select the suitable organogenesis, the explants of the three cultivars were cultured on MS medium supplemented with different concentrations of TDZ (1, 2, 3, and 4 mg/l), then incubated at a temperature of 22 °C ± 2. Medium containing 2 mg/l TDZ revealed the best regeneration efficiency with the three cultivars (72% for Festival, and 73% for Sweet Charly and Florida). After 4 weeks, the produced shoots were cultured on MS medium with different concentrations of BA and Kin to enhance shoot elongation. Results showed that the medium containing 1.5 mg/l BA and 0.5 mg/l Kin revealed highest elongation efficiency (88% and 94%) for Festival and Sweet Charly, respectively. On the other hand, medium containing 1.5 mg/l BA and 0.1 mg/l Kin showed highest elongation efficiency (90%) in Florida. Elongated shoots were successfully rooted on MS medium containing 1.5 mg/l NAA. Furthermore, transformation of the two cultivars, Festival and Sweet Charly, has been established via Agrobacterium strain LBA44404 containing the plasmid pISV2678 with gus-intron and bar genes. Three days post co-cultivation, GUS activity was screening using the histochemical assay. The results showed 16% and 18% of the tested plant materials has changed into blue color for Festival and Sweet Charly, respectively. Out of 120 explants only 13 shoots were developed on bialaphos medium for each cultivar, representing 10.8% bialaphos resistant strawberry shoot. The presence of the both genes bar and uid A was detected by PCR and Northern giving a transformation efficiency of 5%.
Keywords: agrobacterium, direct organogenesis, Fragaria sp., plant transformation, strawberry
Introduction
The cultivated strawberry (Fragaria × ananassa Duch.), a member of the Rosaceae, is an economically important berry crop with high demand for fresh fruits and processing industry.1The global strawberry production is nearly 4.1 million metric tons from almost 255 thousand hectare (FAO 2008). Loss in Strawberry production are caused by several factors which involve a complex interaction between a biotic factors (temperature, soil type, and moisture), and pathogenic infections.
The handiness of regeneration system coupled with the ability of infection by Agrobacterium species2 makes the strawberry well suited for Agrobacterium-mediated genetic transformation. This allows introducing novel gene(s) with new desired traits in strawberry plants via Agrobacterium mediated strategy.
The application of in vitro technique and genetic engineering could be implemented to overcome the limited potential of strawberry improvement through traditional breeding methods.3An efficient in vitro shoot proliferation and regeneration systems, that used in conjunction with classical breeding methods could accelerate cultivar development programs.4,5 Several developments of the in vitro techniques are important tools for modern plant improvement programs that introduce new traits into selected plants, clonal multiplication, germplasm improvement and gene conservation of this flavorful and nutritious berry crop, which may develop suitable cultivars in the minimum time.6,7 Plant regeneration is a crucial aspect of plant biotechnology and tissue culture techniques that facilitates the production of genetically engineered plants, somaclonal variants, and the rapid multiplication of difficult-to-propagate species.5,7
Establishment of an efficient regeneration system for each strawberry cultivar is an essential pre-requisite for the successful development of transgenic plants. Adventitious buds and shoots regeneration has been previously reported from different explants such as: leaves,8-16 petioles,11,12,15,17,18 peduncles/peduncular base of the flower buds,17,18 stems,19 stipules,11,20 stolon,18 roots,11,20 runners9and anther cultures.21 Furthermore, shoot regeneration was produced directly from field,10 greenhouse-grown strawberry plants12,15,22 or from in vitro-grown shoots.1,11
Transformation in strawberries has been previously documented using different constructs in various genotypes.23-27 Strawberry genetic transformation has done outstanding progress. The transformation of Fragaria was focused on establishing the system and using genes with potential for pest and herbicide resistance, cold tolerance and ripening.22,28-36 The aim of this study was to develop an efficient and reproducible Agrobacterium-mediated protocol for the strawberry cultivars using the juvenile leaf explants.
Results and Discussion
Optimization of strawberry regeneration
Plant regeneration from adventitious strawberry explants are accomplished through: (1) formation of viable adventitious shoots from the explant; (2) elongation of the shoots; (3) rooting of the shoots to form whole plants.
Adventitious shoots formation
Pieces from fully juvenile leaves and petioles of 3–4 wk-old from in vitro cultures were used as explants. It was reported that the explants taken from field-grown plants are difficult to sterilize in order to establish in vitro cultures due to high degree of contamination. Thus, it is usually recommended to take explants from plants grown under controlled conditions such as growth room, greenhouse, or from buds which flush from dormant shoots stored indoors.7,37
To evaluate the best media for the shoot formation, the juvenile leaf explants of the three cultivars (Festival, Sweet Charly and Florida) were cultured on MS medium with TDZ at concentration of 1, 2, and 3 mg/l, individually. Cultures were incubated at dark for a week, then, transferred to light for at least three weeks. It was observed that shoots started to develop 10 d post culturing. Four weeks later the produced shoots were ready for transferring into the elongation medium. Medium containing 2 mg/l TDZ produced the highest regeneration efficiency among the three cultivars. Sweet Charly recorded the highest percentage of regeneration response among the three cultivars (73.3%) followed by 71.6% for Festival and Florida (Table 1; Figure 1). In addition, juvenile leaves of Sweet Charly reached the highest number of shoots per explant (4 shoots /explant) compared with other cultivars under study. These results are in consensus with previous reports38,38 indicating that chemical composition of the media is one of the factors determining the success of in vitro. In addition, selection of the proper hormones is the key of regeneration success in strawberry.40-43
Table1. Effect of different TDZ concentration on adventitious shoot regeneration development of the three strawberry cultivars (Festival, Sweet Charly, and Florida).
| TDZ Concentration (mg/L) | Total number of explants | Festival | Sweet Charly | Florida |
|---|---|---|---|---|
| % of shoots | ||||
| 1 | R1 = 100 | 13 | 20 | 10 |
| R2 = 100 | 12 | 19 | 13 | |
| R3 = 100 | 11 | 19 | 15 | |
| Mean: 100 | 12 | 19.3 | 12.7 | |
| 2 | R1 = 100 | 72 | 74 | 70 |
| R2 = 100 | 71 | 73 | 72 | |
| R3 = 100 | 72 | 73 | 73 | |
| Mean: 100 | 71.6 | 73.3 | 71.6 | |
| 3 | R1 = 100 | 39 | 48 | 25 |
| R2 = 100 | 38 | 45 | 23 | |
| R3 = 100 | 37 | 45 | 22 | |
| Mean: 100 | 38 | 46 | 23.3 | |
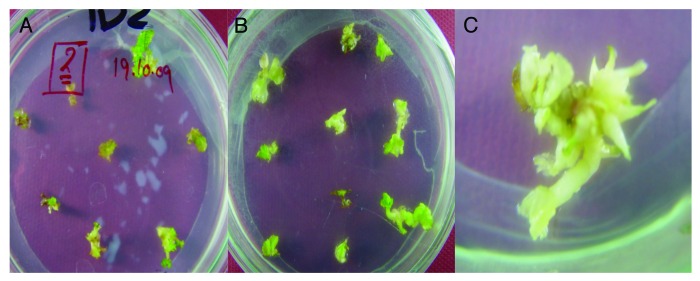
Figure 1. Direct shoots regeneration from the juvenile leaf explants after, (A) 10 d, (B) 3weeks and, (C) 4 wk.
Organogenesis relies on the inherent plasticity of plant tissues and is regulated by altering the components of the medium. It is well known that to induce shoot formation we need to increase the cytokinin to auxin ratio of the culture medium. Thidiazuron (TDZ), as a substituted to phenylurea (Nphenyl- N'-1,2,3-thidiazol-5-ylurea) was used with its cytokinin- and auxin-like effects, is now among the most active cytokinin-like substances for plant tissue culture and has been used to induce shoot organogenesis of strawberries.12 Either TDZ alone12 or in combination with 2,4-dichlorophenoxy-acetic acid (2,4-D)11 or (indol-butyric acid) IBA14 were found to be effective in strawberry shoot regeneration when leaves are the explants.
Incubation in dark is well- known44 to solve the problem of the browning of the surface of plant tissues of in vitro cultured strawberry explants. Browning is produced from the oxidation of phenolic compounds that produce the quinones which reacts with plant tissues leading to tissue poisonous. Therefore, dark incubation is recommended for reducing the tissue browning by inhibiting enzymatic activity that responsible for tissue oxidation.45,46 In strawberry, dark incubation of leaf explants for at least a week is required to enhance organogenesis in strawberry explants.9,20,22,49-51
Elongation of the shoots
After shoot development, produced shoots in cluster were transferred to different elongated media and incubated for 4 wks. The most efficient elongation medium was selected upon reaching shoots height to approximately 1 cm. Results showed that medium SE6 containing 1.5 mg/l 6-benzyladenine (BA) and 0.5 mg/l kinetin (Kin) was the best for elongated shoots in both Festival and Sweet Charly, while SE5 containing 1.5 mg/l BA and 0.1 mg/l Kin showed the best elongation in Florida. (Table 2; Fig. 2). It was known that cytokinins including BA and kinetin play an integral role in controlling the adventitious shoot formation, promote cell division and leaf cell enlargement that stimulate of leaf expansion. Our result indicates that the BA with a concentration of 1.5 mg/l combined with low concentration of kin (0.1 and 0.5 mg/l) have a positive impact in elongation and proliferation of strawberry plants. It was previously reported that the BA was comparatively more effective than Kin in proliferation of shoots. Similarly, Debnath,52 and Haddaidi et al.,53 reported that high concentration of cytokinin with no or law auxin concentration promotes the branching of lateral buds from leaf axis. On other hand, in contrary to our result, Biswas et al.,54 recorded that low concentration (0.1 mg/l) of BA is more effective for mass propagation in the studied strawberries. Debnath15reported that the success in shoots proliferation and roots development was observed when explants were cultured in medium supplemented with zeatin in cultivar Bounty
Table 2. Effect of the different media composition on strawberry shoots elongation.
| Media | Festival | Sweet Sherry | Florida | |||
|---|---|---|---|---|---|---|
| Elongated shoots |
% | Elongated shoots |
% | Elongated shoots |
% | |
| SE1 | 24 | 48 | 20 | 40 | 19 | 38 |
| SE2 | 29 | 58 | 23 | 46 | 25 | 50 |
| SE3 | 32 | 64 | 31 | 62 | 34 | 68 |
| SE4 | 35 | 70 | 37 | 74 | 33 | 66 |
| SE5 | 39 | 78 | 42 | 84 | 45 | 90 |
| SE6 | 44 | 88 | 47 | 94 | 42 | 84 |
| SE7 | 36 | 72 | 31 | 62 | 33 | 66 |
| SE8 | 25 | 50 | 27 | 54 | 23 | 46 |
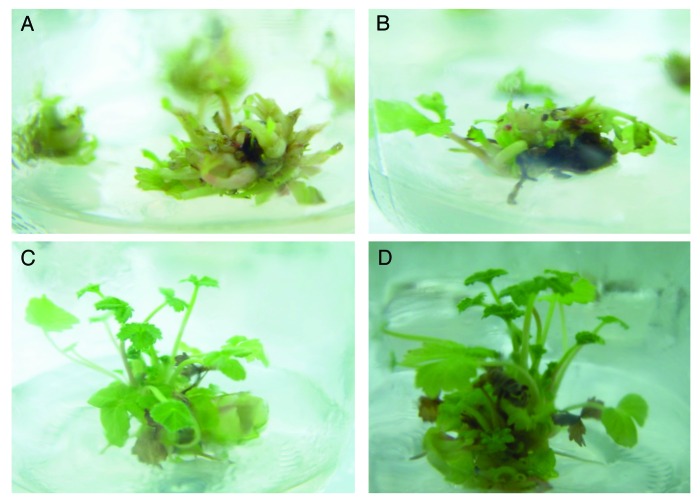
Figure 2. Elongation stage at (A) zero incubation time, (B) one week, (C) 3 wk and (D) 4 wk
Rooting stage of the produced shoots
In order to study root formation, the elongated shoots were transferred to different rooting media. Roots started to appear after about 10 d and complete formation during one month. It was shown that the in vitro-formed roots were thick, possess no hairy roots, grow horizontally. It was observed that the frequency of the rooted shoots number increased when the concentration of IBA and NAA was increased. In addition, the highest concentration of either IBA or NAA which is 1.5 mg/l, revealed the highest percentage of forming roots. Furthermore, IBA revealed higher response than NAA. Among the three cultivars, Festival showed the highest response (Table 3; Fig. 3).
Table 3. The percentage of rooted shoots on different auxins concentrations after 4 wk.
| Hormone | Concentration (mg/l) |
Festival | Sweet Charly | Florida | |||
|---|---|---|---|---|---|---|---|
| No of rooted shoots | % | No of rooted shoots | % | No of rooted shoots | % | ||
| IBA | 0.5 | 18 | 60 | 17 | 56 | 15 | 50 |
| 1.0 | 23 | 77 | 22 | 73 | 22 | 37 | |
| 1.5 | 27 | 90 | 26 | 86 | 24 | 80 | |
| NAA | 0.5 | 13 | 43 | 15 | 50 | 17 | 56 |
| 1.0 | 21 | 70 | 17 | 56 | 15 | 50 | |
| 1.5 | 24 | 80 | 19 | 63 | 21 | 70 | |
Figure 3. Rooted strawberry shoot before and after incubation for 4 wk on rooting medium
In previous articles, it is recommended using a single step for shoot multiplication and rooting which takes place in the same culture medium.15,50 Different media composed of MS with or without auxins that have been used for shoot proliferation in strawberry produced roots at the same time.55 Auxin free half-strength MS medium55 with activated charcoal at concentration of 0.6 g/l and IAA at concentration of 1 mg/l have been also used.56 Recent study using IBA proved superior to (1-Naphthaleneacetic acid (NAA) in root formation frequency. Positive effect of IBA in root formation was also reported in case of Centellasiatica57 and Acacia sinuate.58 On the other hand, Ying et al.59 reported that roots was successfully formed at low concentration of NAA (0.02mg/l). Zeatin also is recommended as a suitable hormone for root development.16
Plant transformation
After optimizing in vitro regeneration of strawberry cultivars, two of them (Sweet Charly and Festival) were subsequently used for establishing Agrobacterium-mediated transformation. Genetic transformation via Agrobacterium with different constructs in various strawberry genotypes has been previously reported.23-27,60In the present study, Agrobacterium mediated transformation of strawberry plants has been established using the juvenile leaf as explant and the plasmid pISV2678 harbors the gus–intron and bar genes. Transformation in strawberries has been performed in two independent experiments with a total of 120 explants for each cultivar (Sweet Charly and Festival).Explants were soaked O/N Agrobacterium suspension for 5and 10 min; co-cultivated for three days on selected regeneration medium and then transferred onto the selective medium (regeneration medium with 1.5 mg/l bialaphos and 300mg/l carbincillin). It was observed that explants which were immersed in the Agrobacterium culture for 10 min were more efficient for regeneration than 5 min (Data not shown). After about 15 d, a number of 22 out of 120 explants of Festival and 27 out of 120 explants of cultivar Sweet Charly were bialaphos resistant. Six weeks further, the bialaphos resistant explants produced only 13 shoots developed from each cultivar. Shoots were subsequently transferred to the elongation medium with 300 mg/l carbincillin for 4 wk and then transferred to the rooting medium for at least four weeks (Table 4).
Table 4. Transformation efficiency of juvenile leave explants of two strawberry cultivars (Festival and Sweet Charly).
| Festival | Sweet Charly | ||||
|---|---|---|---|---|---|
| Total No. of explants | No. of survived explants | Produced shoots | Total No. of explants | No. of survived explants | Produced shoots |
| 60 | 10 | 5 | 60 | 12 | 7 |
| 60 | 12 | 8 | 60 | 15 | 6 |
| Total (120) | 22 | 13 | Total (120) | 27 | 13 |
| % of survived explants | 18.3 | % of survived explants | 22.5 | ||
| % of produced shoots | 10.8 | % of produced shoots | 10.8 | ||
Rapid shoot regeneration is requested to minimize the risks that are associated with strawberry transformation including escapes, formation of chimeric shoots and somaclonal variation.7,61In this work, the regeneration period took only 12 to 14 wk, which is less than the previously reported protocols with about 2 wks.60
GUS activity screening was performed three days post co-cultivation using the histochemical assay in treated plant leaves. Results showed that 16% and 18% of the tested plant materials stained with blue color for Festival and Sweet Charly, respectively (Fig. 4).
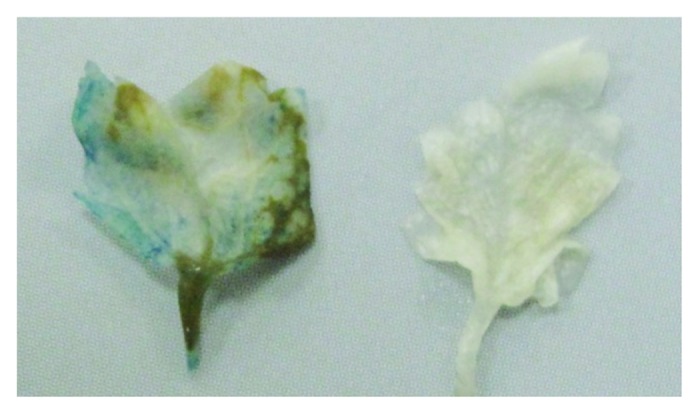
Figure 4. GUS assay of treated leaf explants (left) and control strawberry leaves (right)
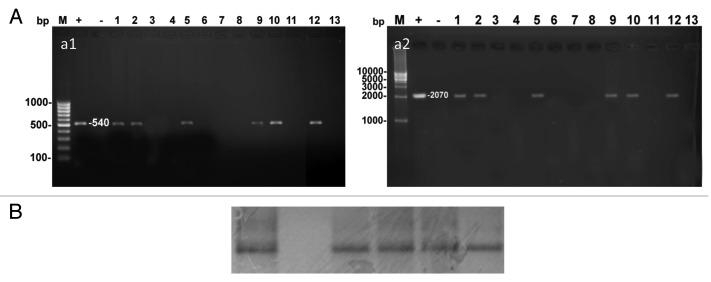
The strawberry plants with positive expression of GUS were further confirmed for transgene (bar and gus genes) integration by PCR. The expected 540 bp and 2070 bp bands corresponding to bar and uid A genes, respectively, were detected in six plants out of 13 putatively transgenic shoots (representing 46% transgenic plants) with Festival and Sweet Charly (Fig. 5A1 and 5A2). In order to make clear if there is any relation between the level of expression of gus gene and the level of transcription, Northern hybridization was done with GUS positive plants and transcription level as can be seen from Figure 5B.
Figure 5. (A) PCR analysis of putative transgenic strawberry plants (Lanes 1–13) using, a1) bar-specific primers amplifying 540 bp and a2) gus-specific primers amplifying 2070 bp. (B) northern blot analysis to detect the presence of gus transcription in transformed strawberry plants.
The transformation efficiency was calculated as the number of bialaphos resistant strawberry plants developed on separate shoots with respect to the total number of explants infected, which was 10.8% for both cultivars. However, the transformation efficiency based on the PCR results with respect to a total number of infected explants recorded 5%. Our obtained protocol for strawberry transformation via Agrobacterium is in the same consensus as previously protocols with different cultivars with transformation ratio ranged between 4–11% among different cultivars and transformation protocols. A transformation frequency of 0.95% has been reported for the cultivar ‘Rapella’,62 6.5% for ‘Red Coat’30,31 and efficiency of 4.22% and 10% with cultivar Chandeler,22,60and 11% for ‘Firework’.63
Materials and Methods
Plant materials
Strawberry cultivars, Festival, Sweet Charly and Florida were used in this study; these cultivars are kindly provided as in vitro cultures from Agricultural Modern Company (PICO). All cultures were incubated at 22 °C ± 2 °C with a 16 h photoperiod under white fluorescent lamps or at dark in a growth room.
Bacteria and plasmid
The Agrobacterium tumefaciens strain LBA4404 that harboring the plasmid pISV2678 with the gus–intron under the control of 35S promoter and nos terminator as well as the bar fused the AMV Leader gene under the control of nos promoter, pAg7 terminator64 was used for optimizing the transformation system in strawberry via Agrobacterium-mediated transformation.65 The plasmid was kindly provided by Dr. P. Ratet, Institut des Sciences Vegetales (ISV), Centre National de la Recherche Scientifique (CNRS), Gif-Sur-Yvette, France.
Plant regeneration
Regeneration system of strawberry has been developed using in vitro juvenile leaf as explants. Explants were collected for the three cultivars, i.e., Festival, Sweet Charly, and Florida, from the in vitro cultures on propagation medium which composed of MS basal medium (Murashige and Skoog,66 supplemented with 0.5 mg/l BA; 0.1 mg/l gibberelic acid (GA3) and 0.1 mg/l IBA as described by Boxus67 and Litwińczuk.42
In order to optimize the proper regeneration medium, the explants were cultured on solid MS medium supplemented with different concentrations (1, 2, 3, and 4 mg/l) of Thidiazuron (TDZ).The cultures were incubated at the growth chamber under dark for one week and then transferred to the light condition. This experiment was repeated three times each time has a number of 100 explants. Data were analyzed based upon the percentage of means.
Subsequently, the produced 4-wk-old shoots in clusters from the previous stage were transferred to the medium for elongating and proliferating. To select the best elongation medium, different concentration and combination of the hormones BA and Kin were tested (Table 5). Data were analyzed based upon the percentage of means.
Table 5. Composition of different elongation media.
| Media | Hormone concentration | |
|---|---|---|
| BA (mg/l) | Kin (mg/l) | |
| SE1 | 0.5 | 0.1 |
| SE2 | 0.5 | 0.5 |
| SE3 | 1.0 | 0.1 |
| SE4 | 1.0 | 0.5 |
| SE5 | 1.5 | 0.1 |
| SE6 | 1.5 | 0.5 |
| SE7 | 2.0 | 0.1 |
| SE8 | 2.0 | 0.5 |
Four weeks further on elongation stage, shoot heights of 1 cm approximately were excised and transferred to different rooting media based on MS basal medium with naphthalene acetic acid (NAA) and IBA in different concentration, 0.5, 0.1, and 1.5 mg/l, individually, and incubated for 3 wk. Each treatment has a total number of 30 shoots. Data were analyzed based upon the percentage of means.
Transformation system of strawberry
Primary culture of Agrobacterium was prepared by inoculating single colony from a freshly streaked plate, in 5 ml of autoclaved liquid YEP medium supplemented with 25 mg/l streptomycin, 10 mg/l rifampicin, 50 mg/l kanamycin. The culture was incubated for overnight in dark at 28 °C. Subculture was performed in a 100 ml YEP medium supplemented with same antibiotics grown under similar conditions. Once the OD600 reached 0.8, Agrobacterium cells were pelleted by centrifugation and were resuspended in MS re-suspension medium containing 150 μM acetosyringone to adjust the O.D.600 of the bacterial suspension to ~0.3. In vitro juvenile leaf explants of the two cultivars (Festival and Sweet Charly) were soaked in the Agrobacterium culture for 5, 10 min. This was performed through two replicates and the numbers of explants in each replicate were 60 with a total number of 120 explants. The treated explants were cultured onto selected regeneration medium for 3 d. Subsequently, they were transferred to the selection medium (regeneration medium supplemented with1.5 mg/l bialaphos and 300 mg/L carbinicillin) and incubated for 2–3 wk. Developed shoots were excised from their original explants and then transferred to the root formation medium. Plantlets were then acclimatized in the greenhouse.
Molecular confirmation of putative transgenic plants
Histochemical GUS assay
GUS expression was visually determined using the histochemical assay according to Jefferson et al.68 The GUS reaction was performed three day post transformation by incubating the samples with GUS buffer solution containing 1mM X-gluc (5-bromo-4-choloro-3-indolyl-β-glucuronic acid) overnight at 37 °C. The blue color was detected visually by naked eye and using light microscope after bleaching the chlorophyll and fixation in 70% ethanol.
PCR
To confirm the presence of transgenes in bialaphos resistant plants, these plants along with wild type (WT) lines were analyzed through PCR analysis. Total genomic DNA from various independent young leaves of putative transgenic plants was extracted as described by Dellaporta et al.69 and was used for PCR using the gus specific primers (forward 5′AACAATGCGCGGTGGTCAGTCCC3′and reverse 5′ATTCATTGTTTGCCTCCCTGCTGC3′)designed to amplify the full gus-intron of 2070bpand the bar specific primers (forward 5′CCACCATGAGCCCAGAACGACG3′and reverse 5′TCAGATCTCGGTGACGG3′)designed to amplify 540 bp. The PCR products were analyzed on 1% agarose gel containing ethidium bromide and visualized under gel documentation unit.
The PCR reactions were performed in a total volume of 25 µl, containing 1 μl DNA, 20 pmol of each primer, 200 μM each dNTP, 0.5 unit Taq DNA polymerase and 3 μl 10x PCR buffer. The PCR temperature profile was as follows: initial denaturation of DNA at 94 °C for 5 min, 35 cycles comprised of 1 min denaturation at 94 °C, 1 min annealing at 55 °C, extension for2 min at 72 °C followed by a final extension step at 72 °C for 7 min. Amplification products were analyzed by electrophoresis on 1% agarose gels and detected by staining with ethidium bromide.
Northern hybridization of transgenic strawberry plants
Total RNA was isolated from various transgenic plants using a modification of a CTAB-based method and was separated with electrophoresis in 1.2% denaturing gel then transferred to Hybond nylon membrane for Northern hybridization.70
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work has been funded by Science and Technology Development Fund (STDF) for funding this investigation under a project entitled “Developing strawberry plants resistant to whitefly transmitted geminivirus using siRNA strategy.”
References
- 1.Shulaev V, Korban SS, Sosinski B, Abbott AG, Aldwinckle HS, Folta KM, Iezzoni A, Main D, Arús P, Dandekar AM, et al. . Multiple models for Rosaceae genomics. Plant Physiol 2008; 147:985 - 1003; http://dx.doi.org/ 10.1104/pp.107.115618; PMID: 18487361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uratsu SL, Ahmadi H, Bringhurst RS, Dandekar AM. . Relative virulence of Agrobacterium strains on strawberry. HortScience 1991; 26:196 - 9 [Google Scholar]
- 3.Husaini AM, Srivastava DK. . Genetic transformation in strawberry – Areview. Asian Journal of Microbiology, Biotechnology, and Environmental Sciences 2006; 8:75 - 81 [Google Scholar]
- 4.Cao X, Hammerschlag FA. . Improved shoot organogenesis from leaf explants of high bush blueberry. HortScience 2000; 35:945 - 7 [Google Scholar]
- 5.Husaini AM, Mercado JA, Teixeira da Silva JA, Schaart JG. . Somatic Embryogenesis and Agrobacterium tumefaciens-Mediated Transformation of Strawberry. Genes, Genomes and Genomics 2011; 5:1 - 11 [Google Scholar]
- 6.Taji A, Kumar PP, Lakshmanan P. In Vitro Plant Breeding, Food Pro ducts Press, New York, 2002; 167 pp. [Google Scholar]
- 7.Debnath SC, Teixeira da Silva JA. Strawberry Culture In Vitro: Applications in Genetic Transformation and Biotechnology, Fruit, Vegetable and Cereal Science and Biotechnology 2007; Global Science Books. [Google Scholar]
- 8.Nehra NS, Stushnoff C, Kartha KK. . Regeneration of plants from immature, leaf-derived callus of strawberry. HortScience 1988; 23:756 [Google Scholar]
- 9.Liu ZR, Sanford JC. . Plant regeneration by organogenesis from strawberry leaf and runner culture. HortScience 1988; 23:1056 - 9 [Google Scholar]
- 10.Nehra NS, Stushnoff C, Kartha KK. . Direct shoot regeneration from strawberry leaf disks. J Am Soc Hortic Sci 1989; 114:1014 - 8 [Google Scholar]
- 11.Passey AJ, Barrett KJ, James DJ. . Adventitious shoot regeneration from seven commercial strawberry cultivars (Fragaria x ananassa Duch.) using a range of explant types. Plant Cell Rep 2003; 21:397 - 401; PMID: 12789440 [DOI] [PubMed] [Google Scholar]
- 12.Debnath SC. . Strawberry sepal: another explant for thidiazuron-induced adventitious shoot regeneration. In Vitro Cell Dev Biol Plant 2005; 41:671 - 6; http://dx.doi.org/ 10.1079/IVP2005688 [DOI] [Google Scholar]
- 13.Qin Y, Zhang S, Asghar S, Zhang LX, Qin Q, Chen K, Xu C. . Regeneration mechanism of Toyonoka strawberry under different color plastic films. Plant Sci 2005; 168:1425 - 31; http://dx.doi.org/ 10.1016/j.plantsci.2004.11.016 [DOI] [Google Scholar]
- 14.Hanhineva K, Kokko H, Karenlampi S. . Shoot regeneration from leaf explants of five strawberry (Fragaria × Ananassa) cultivars bioreactors systems. In Vitro Cell Dev Biol Plant 2005; 41:826 - 31; http://dx.doi.org/ 10.1079/IVP2005714 [DOI] [Google Scholar]
- 15.Debnath SC. . Zeatin overcomes thidiazuron-induced inhibition of shoot elongation and promotes rooting in strawberry culture in vitro. J Hortic Sci Biotechnol 2006; 81:349 - 54 [Google Scholar]
- 16.Husaini AM, Abdin MZ. . Interactive effect of light, temperature and TDZ on the regeneration potential of leaf discs of Fragaria × ananassa Duch. In Vitro Cell Dev Biol Plant 2007; 43:576 - 84; http://dx.doi.org/ 10.1007/s11627-007-9048-3 [DOI] [Google Scholar]
- 17.Foucault C, Letouze R. . In vitro regeneration de plantes de Fraisier a partir de fragmentes de petiole et de bourgeons floraux. Biol Plant 1987; 29:409 - 14; http://dx.doi.org/ 10.1007/BF02882207 [DOI] [Google Scholar]
- 18.Lis EK. . Strawberry plant regeneration by organogenesis from peduncle and stolon segements. Acta Hortic 1993; 348:435 - 8 [Google Scholar]
- 18.Rugini E, Orlando R. . High efficiency shoot regeneration from calluses of strawberry (Fragaria × ananassa Duch.) stipules of in vitro cultures. J Hortic Sci 1992; 67:577 - 82 [Google Scholar]
- 19.Graham J, McNicol RJ, Grieg K. . Towards genetic based insect resistance in strawberry using the Cowpea tryps in inhibitor gene. Ann Appl Biol 1995; 127:163 - 73; http://dx.doi.org/ 10.1111/j.1744-7348.1995.tb06661.x [DOI] [Google Scholar]
- 20.Rugini E, Orlando R. . High efficiency shoot regeneration from callus of strawberry (Fragaria × ananassa Duch.) stipules of in vitro cultures. J Hortic Sci 1992; 67:577 - 82 [Google Scholar]
- 21.Owen HR, Miller AR. . Haploid plant regeneration from anther cultures of three north american cultivars of strawberry (Fragaria x ananassa Duch.). Plant Cell Rep 1996; 15:905 - 9; http://dx.doi.org/ 10.1007/BF00231585; PMID: 24178272 [DOI] [PubMed] [Google Scholar]
- 22.Barceló M, El-Mansouri I, Mercado JA, Quesada MA, Alfaro FP. . Regeneration and transformation via Agrobacterium tumefaciens of the strawberry cultivar Chandler. Plant Cell Tissue Organ Cult 1998; 54:29 - 36; http://dx.doi.org/ 10.1023/A:1006031527413 [DOI] [Google Scholar]
- 23.Cordero de Mesa M, Jimenez-Bermudez S, Pliego-Alfaro F, Quesada MA, Mercado JA. . Agrobacterium cells as microprojectile coating: a novel approach to enhance stable transformation rates in strawberry. Aust J Plant Physiol 2000; 27:1093 - 100 [Google Scholar]
- 24.Zhao Y, Liu Q, Davis RE. . Transgene expression in strawberries driven by a heterologous phloem-specific promoter. Plant Cell Rep 2004; 23:224 - 30; http://dx.doi.org/ 10.1007/s00299-004-0812-0; PMID: 15235813 [DOI] [PubMed] [Google Scholar]
- 25.Khammuang S, Dheeranupattana S, Hanmuangjai P, Wongroung S. . Agrobacterium-mediated transformation of modified antifreeze protein gene in strawberry. Songklanakarin Journal of Science and Technology 2005; 27:693 - 703 [Google Scholar]
- 26.Park J-I, Lee Y-K, Chung W-I, Lee I-H, Choi J-H, Lee W-M, Ezura H, Lee S-P, Kim I-J. . Modification of sugar composition in strawberry fruit by antisense suppression of an ADP-glucose pyrophosphorylase. Mol Breed 2006; 17:269 - 79; http://dx.doi.org/ 10.1007/s11032-005-5682-9 [DOI] [Google Scholar]
- 27.Vellicce GR, Ricci JCD, Hernández L, Castagnaro AP. . Enhanced resistance to Botrytis cinerea mediated by the transgenic expression of the chitinase gene ch5B in strawberry. Transgenic Res 2006; 15:57 - 68; http://dx.doi.org/ 10.1007/s11248-005-2543-6; PMID: 16475010 [DOI] [PubMed] [Google Scholar]
- 28.Jelenkovic G, Chin CK, Billings S. . Transformation studies of Fragaria× ananassa by Ti-plasmids of Agrobacterium tumefaciens.. [Abstract] HortScience 1986; 21:695 [Google Scholar]
- 29.James DJ, Passey AJ, Barbara DJ. . Agrobacterium-mediated transformation of the cultivated strawberry (Fragaria×ananassaDuch.) using disarmed binary vectors. Plant Sci 1990; 69:79 - 94; http://dx.doi.org/ 10.1016/0168-9452(90)90106-X [DOI] [Google Scholar]
- 30.Nehra NS, Chibbar RN, Kartha KK, Datla RSS, Crosby WL, Stushnoff C. . Genetic transformation of strawberry by Agrobacterium tumefaciens using a leaf disk regeneration system. Plant Cell Rep 1990; 9:293 - 8; http://dx.doi.org/ 10.1007/BF00232854; PMID: 24226936 [DOI] [PubMed] [Google Scholar]
- 31.Nehra NS, Chibbar RN, Kartha KK, Datla RSS, Crosby WL, Stushnoff C. . Agrobacterium-mediated transformation of strawberry calli and recovery of transgenic plants. Plant Cell Rep 1990; 9:10 - 3; http://dx.doi.org/ 10.1007/BF00232125; PMID: 24226368 [DOI] [PubMed] [Google Scholar]
- 32.El Mansouri I, Mercado JA, Valpuesta V, López-Aranda JM, Pliego-Alfaro F, Quesada MA. . Shoot regeneration and Agrobacterium-mediated transformation of Fragaria vesca L. Plant Cell Rep 1996; 15:642 - 6; http://dx.doi.org/ 10.1007/BF00232469; PMID: 24178534 [DOI] [PubMed] [Google Scholar]
- 33.Haymes KM, Davis TM. . Agrobacterium-mediated transformation of ‘Alpine’ Fragariavesca, and transmission of transgenes to R1 progeny. Plant Cell Rep 1998; 17:279 - 83; http://dx.doi.org/ 10.1007/s002990050392 [DOI] [PubMed] [Google Scholar]
- 34.Ricardo YG, Coll Y, Castagnaro A, Diaz Ricci JC. . Transformation of strawberry cultivar using a modified regeneration medium. HortScience 2003; 38:277 - 80 [Google Scholar]
- 35.Folta KM, Dhingra A, Howard L, Stewart PJ, Chandler CK. . Characterization of LF9, an octoploid strawberry genotype selected for rapid regeneration and transformation. Planta 2006; 224:1058 - 67; http://dx.doi.org/ 10.1007/s00425-006-0278-0; PMID: 16614818 [DOI] [PubMed] [Google Scholar]
- 36.Mezzetti B, Costantini E. . Strawberry (Fragaria x ananassa). Methods Mol Biol 2006; 344:287 - 95; PMID: 17033071 [DOI] [PubMed] [Google Scholar]
- 37.Gerdakaneh M, Mozafari AA, Khalighi A, Sioseh-mardah A. . The effects of carbohydrate source and concentration on somatic embryogenesis of strawberry (Fragaria × ananassaDuch.). Am Eurasian J Agric Environ Sci 2009; 6:76 - 80 [Google Scholar]
- 38.Kozak D, Hetman J, Witek M.. . The influence of the mineral composition of the medium on in vitro propagation of kohleriaamabilis Fritsch shoots. ACTA AGROBOTANICA 2007; 60:95 - 99; http://dx.doi.org/ 10.5586/aa.2007.011 [DOI] [Google Scholar]
- 39.Faedi W, Mourgues F, Rosati C. . Strawberry breeding and varieties: situation and perspectives. Acta Hortic 2002; 567:51 - 9 [Google Scholar]
- 40.Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, Pliego-Alfaro F, Quesada MA, Mercado JA. . Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol 2002; 128:751 - 9; http://dx.doi.org/ 10.1104/pp.010671; PMID: 11842178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morozova T. . Genetic stability of pure line of (Fragaria vesca L.) in micro-propagated and long term storage in vitro. Acta Hortic 2003; 567:85 - 8 [Google Scholar]
- 42.Litwińczuk W.. . Field performance of ‘sengasengana’ strawberry plants (Fragaria × ananassa duch.) Obtained by runners and in vitro through auxiliary and adventitious shoots. Electronic Journal of Polish Agricultural Universities, Horticulture 2004; 7:1 [Google Scholar]
- 43.Mozafari AA, Gerdakaneh M. . Influence of media and growth regulators on regeneration and morphological characteristics of strawberry cvs Kurdistan and Merck (Fragaria x ananassaDuch.). International Journal of Plant Physiology and Biochemistry 2012; 4:99 - 104 [Google Scholar]
- 44.Taji AM, Williams RR. Overview of Plant Tissue Culture. In: Taji AMand Williams RR (Eds) Tissue Culture of Australian Plants: Past, Present and Future, University of New England Press, Armidale, Australia, 1996; pp 1-15. [Google Scholar]
- 45.George EF. Plant Propagation by Tissue Culture, Part 1: In Practice, Exegetics Ltd., Edington, UK, 1993; 574 pp. [Google Scholar]
- 46.Titov S, Bhowmik SK, Mandal A, Alam MS, Nasiruddin S. . Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. Kanthali floral bud explants. American Journal of Biochemistry and Biotechnology 2006; 2:97 - 104; http://dx.doi.org/ 10.3844/ajbbsp.2006.97.104 [DOI] [Google Scholar]
- 47.Nehra NS, Stushnoff C, Kartha KK. . Direct shoot regeneration from strawberry leaf disks. J Am Soc Hortic Sci 1989; 114:1014 - 8 [Google Scholar]
- 48.Blando F, Niglio A, Frattarelli A. . Cell suspension cultures in strawberry: Growth characterization and variability. Acta Hortic 1993; 336:257 - 62 [Google Scholar]
- 49.Popescu AN, Isac VS, Coman MS, Radulescu MS. . Somaclonal variation in plants regenerated by organogenesis from callus culture of strawberry(Fragaria × ananassa). Acta Hortic 1997; 439:89 - 96 [Google Scholar]
- 50.Husaini AM, Abdin MZ. . Development of transgenic strawberry (Fragaria×ananassa Duch.) plants tolerant to salt stress. Plant Sci 2008; 174:446 - 55; http://dx.doi.org/ 10.1016/j.plantsci.2008.01.007 [DOI] [Google Scholar]
- 51.Landi L, Mezzetti B. . TDZ, auxin and genotype effects on leaf organogenesis in Fragaria.. Plant Cell Rep 2006; 25:281 - 8; http://dx.doi.org/ 10.1007/s00299-005-0066-5; PMID: 16231183 [DOI] [PubMed] [Google Scholar]
- 52.Debnath SC. Micropropagation of small fruits. In: Jain SM, Ishii K(Eds) Micropropagation of Woody Trees and Fruits, Kluwer Academic Publishers, Dordrecht, Germany, 2003; pp 465-506. [Google Scholar]
- 53.Haddadi F, Aziz MA, Saleh G, Rashid AA, Kamaladini H. . Micropropagation of strawberry cv. Camarosa: Prolific shoot regeneration from in vitro shoot tips using thidiazuron with N6-benzylamino-purine. HortScience 2010; 45:453 - 6 [Google Scholar]
- 54.Biswas MK, Islam R, Hossain M. . Micro propagation and field evaluation of strawberry in Bangladesh. Journal of Agricultural Technology 2008; 4:167 - 82 [Google Scholar]
- 55.Yue D, Gosselin A, Desjardins Y. . Re-examination of the photosynthetic capacity of in vitro-cultured strawberry plantlets. J Am Soc Hortic Sci 1993; 118:419 - 24 [Google Scholar]
- 56.Moore PP, Robbins JA, Sjulin TM. . Field performance of ‘Olympus’ strawberry subclones. HortScience 1991; 26:192 - 4 [Google Scholar]
- 57.Banerjee S, Zehra M, Kumar S. . In vitro multiplication of Centella asiatica, a medicinal herb from leaf explants. Curr Sci 1999; 76:147 - 8 [Google Scholar]
- 58.Tiwari KN, Sharma NC, Tiwari V, Singh BD. . Micropropagation of Centella asiatica a valuable medicinal herb. Plant Cell Tissue Organ Cult 2000; •••:61 [Google Scholar]
- 59.Ying CK, Al-Abdulkarim A. . M., Al-Jowid, S. M., and Al-Baiz, A.An effective disinfection protocol for plant regeneration from shoot tip cultures of strawberry. Afr J Biotechnol 2009; 8:2611 - 5 [Google Scholar]
- 60.Husaini AM. . Pre- and post-agroinfection strategies for efficient leaf disk transformation and regeneration of transgenic strawberry plants. Plant Cell Rep 2010; 29:97 - 110; http://dx.doi.org/ 10.1007/s00299-009-0801-4; PMID: 19956955 [DOI] [PubMed] [Google Scholar]
- 61.Mathews H, Dewey V, Wagoner W, Bestwick RK. . Molecular and cellular evidence of chimaeric tissues in primary transgenics and elimination of chimaerism through improved selection protocols. Transgenic Res 1998; 7:123 - 9; http://dx.doi.org/ 10.1023/A:1008872425917 [DOI] [Google Scholar]
- 62.James DJ, Passey AJ, Barbara DJ. . Agrobacterium mediated transformation of apple and strawberry using disarmed Ti-binary vectors. Acta Hortic 1990; 280:495 - 502 [Google Scholar]
- 63.Shestibratov KA, Dolgov SV. . Transgenic strawberry plants expressing athaumatin II gene demonstrate enhanced resistance to Botrytis cinerea.. Sci Hortic (Amsterdam) 2005; 106:177 - 89; http://dx.doi.org/ 10.1016/j.scienta.2005.03.016 [DOI] [Google Scholar]
- 64.Becker D, Kemper E, Schell J, Masterson R. . New plant binary vectors with selectable markers located proximal to the left TDNA border. Plant Mol Biol 1992; 20:1197; http://dx.doi.org/ 10.1007/BF00028908 [DOI] [PubMed] [Google Scholar]
- 65.Horsch RB, Fry JE, Hoffmann NL, Rogers SG, Fraley RT. . A simple and general method for transferring genes into plants. Science 1985; 227:1229 - 31; http://dx.doi.org/ 10.1126/science.227.4691.1229; PMID: 17757866 [DOI] [PubMed] [Google Scholar]
- 66.Murashige T, Skoog F. . A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962; 15:473 - 97; http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 67.Bouxus P. Micropropagation of strawberry of strawberry via axillary shoots proliferation. In R.D. Hall (ed.), Plant cell culture protocols: Methods in molecular biology, Humana Press, Totowa, N.J:1999; pp:103-114. [DOI] [PubMed] [Google Scholar]
- 68.Jefferson RA, Kavanagh TA, Bevan MW. . GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 1987; 6:3901 - 7; PMID: 3327686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dellaporta SL, Wood J, Hicks JB. . A plant DNA miniprepration: version II. Plant Mol Biol Rep 1983; 1:19 - 21; http://dx.doi.org/ 10.1007/BF02712670 [DOI] [Google Scholar]
- 70.Chang S, Puryear J, Cairney J. . Simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 1993; 11:113 - 6; http://dx.doi.org/ 10.1007/BF02670468 [DOI] [Google Scholar]