Abstract
Premise of the study:
Internationally, gardens hold diverse living collections that can be preserved for genomic research. Workflows have been developed for genomic tissue sampling in other taxa (e.g., vertebrates), but are inadequate for plants. We outline a workflow for tissue sampling intended for two audiences: botanists interested in genomics research and garden staff who plan to voucher living collections.
Methods and Results:
Standard herbarium methods are used to collect vouchers, label information and images are entered into a publicly accessible database, and leaf tissue is preserved in silica and liquid nitrogen. A five-step approach for genomic tissue sampling is presented for sampling from living collections according to current best practices.
Conclusions:
Collecting genome-quality samples from gardens is an economical and rapid way to make available for scientific research tissue from the diversity of plants on Earth. The Global Genome Initiative will facilitate and lead this endeavor through international partnerships.
Keywords: arboreta, biorepository, botanic gardens, collection workflow, genomics, voucher preparation
The genomics revolution is rapidly materializing as reduction in the cost per base pair is realized through improvements to high-throughput sequencing platforms, bioinformatics, and general analytical methodology (Glenn, 2011). In preparation for the coming wave of genome sequencing needs, the Smithsonian Institution has established the Global Genome Initiative (GGI; http://www.ggi.si.edu/), which seeks to collect, voucher, and preserve genome-quality tissues from at least one species in each family and 50% of all the genera of life on Earth by 2020. For the green plant branch of the tree of life, GGI has started a program called GGI–Gardens that has as its mission the collection of genome-quality leaf samples, as well as their associated vouchers, from plants housed in gardens, greenhouses, and arboreta (hereafter called “Gardens”). In line with this goal, GGI–Gardens is forming partnerships with Gardens from around the world to form an international consortium of living plant collections that will work together to preserve samples to facilitate scientific research around the world. Here we outline a workflow for the collection and storage of leaf tissue and vouchers to be used by any organization interested in preserving genome-quality tissue for use by current and future generations of researchers. The intended audience for this article includes both botanists who have never collected tissue for genomics work, but wish to do so, and garden staff who plan to voucher their living collections.
Natural history collections serve a critical function for a variety of studies including, but not limited to, biodiversity, conservation, and evolution. For plants, herbaria and their associated collections (i.e., libraries, illustrations, photographs) represent the cornerstone of natural history collections. Currently, there are more than 350,000,000 plant and fungi specimens stored within nearly 3000 herbaria around the world (Thiers, 2016), and this number continues to grow. Herbarium collections enable essential research of plant and fungal diversity, but these collections are often ill-equipped for genomic applications (e.g., whole genome sequencing). Until recently, natural history collections have had few available resources to preserve genome-quality tissues. Zimkus and Ford (2014) surveyed genetic resource curation from 45 natural history collections across 39 institutions and found varying practices in place from sampling acquisition to final storage. Only recently have best practices been developed for repositories (see ISBER, 2012), and in many cases these have been optimized for animal or human biological or environmental samples. For instance, a recent methodology (Wong et al., 2012) identified a four-star standard system for vertebrate genomic tissue sampling; however, although basic collection practices have been identified (see Spooner and Ruess, 2014), no such method or standard exists yet for plants.
There are already in operation several well-funded and globally important programs for the collection and preservation of plant genetic resources, such as the U.S. Department of Agriculture (USDA) Germplasm Resources Information Network (GRIN; http://www.ars-grin.gov/); the Millennium Seed Bank hosted by Kew Gardens (http://www.kew.org/science-conservation/collections/millennium-seed-bank), which has samples of more than 36,000 species (van Slageren, 2003); and the Svalbard Global Seed Vault (http://www.regjeringen.no/en/topics/food-fisheries-and-agriculture/landbruk/svalbard-global-seed-vault), which houses viable, duplicate samples of important crops and their wild relatives (Fowler, 2008). These organizations reflect a global commitment to the preservation of genetic resources with similar, but in some cases fundamentally different purposes (i.e., preserve and share genetic resources of crops and their wild relatives through GRIN). However, there has been no concerted effort to sample plant biodiversity across the globe and store tissue for future use in basic research that harnesses genome sequences. To accommodate this need, the Global Genome Biodiversity Network (GGBN; Seberg et al., 2016) was founded as a consortium of biorepositories to facilitate the storage of genome-quality tissues for natural history collections. GGI, including GGI–Gardens, is part of the GGBN, and the workflow described here is meant for tissues from living plants that are destined for a GGBN facility; however, it can be modified to suit the needs of other preservation methods. The workflow serves as a template for ongoing comparative studies of optimal plant DNA/RNA sampling and preservation techniques for long-term storage and genomic research.
METHODS AND RESULTS
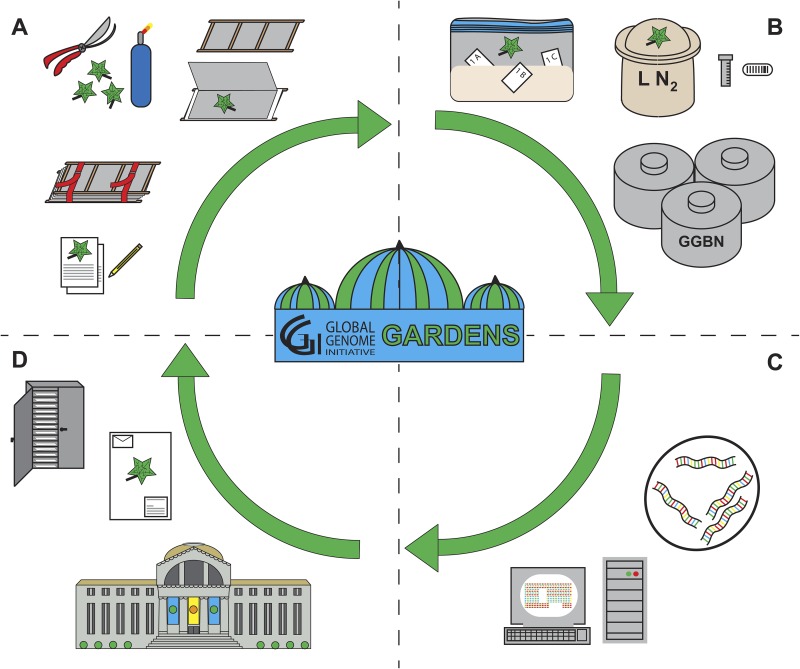
The workflow described here was tested over the course of six months. Collection from sites in 2015 resulted in ca. 900 voucher specimens by teams of two to four individuals. Each team was led by an experienced field biologist to make sure the plants were properly collected and that information taken down in the field was accurate. The remaining members were interns. Participation by a garden staff member is essential for successful and efficient collecting expeditions. During this time, collecting sites included the Smithsonian Institution Gardens and their greenhouses, the Department of Botany (National Museum of Natural History [NMNH]) greenhouse, the U.S. National Arboretum and South Farm (both USDA), and the United States Botanic Garden (Architect of the Capitol) and production facility. For each voucher specimen, tissue suitable for the isolation of DNA/RNA was collected and preserved in two ways: silica gel desiccant and liquid nitrogen. At least two high-quality photographic images were taken (habit and close up) for each specimen, and all collection information and images were uploaded into the NMNH electronic record management database (http://collections.nmnh.si.edu/search/botany/). Collection procedures were ideally situated for teams of three individuals and have been outlined in Appendix 1, which includes a step-by-step protocol for collection needs prior to, during, and immediately following sampling. We describe an “any or all” approach to sampling procedures from living collections in Gardens for genomic research according to five steps: (a) preparation of living collections for genome tissue sampling, (b) voucher specimen handling and collection, (c) genetic sample handling and collection, (d) biorepository handling and storage, and (e) common sampling problems and some solutions. These GGI–Gardens workflow steps are summarized diagrammatically in Fig. 1, and a sample collection sheet is shown in Fig. 2.
Fig. 1.
Diagram of the workflow associated with GGI–Gardens genomic tissue sampling. (A) Genomic tissue sampling begins in a garden or greenhouse. Targeted specimens are collected with pruning shears, and a voucher specimen is prepared using a plant press. Detailed notes and photographs are taken in the field describing the living specimens and their locality. (B) All sampled specimens also contribute tissue for preservation on silica gel and in liquid nitrogen. Silica-preserved tissues are placed into labeled envelopes and kept in a resealable bag filled with silica gel. Liquid nitrogen is brought into the garden/greenhouse in a transportation Dewar flask. Tissues are placed into GGBN-approved cryogenic storage tubes, to which a barcode label is affixed. After wrapping cryo-tubes in aluminum foil, they are deposited into the Dewar and then sent to a biorepository for storage. (C) Preserved and stored genome-quality tissue samples can be accessed for research including whole genome sequencing. Data for taxa sampled by GGI–Gardens can be accessed by searching the GGBN web portal (GGBN, 2011). (D) All voucher specimens are mounted and deposited into a recognized herbarium. Future collections are informed by GGI Gap analyses, which allow GGI–Gardens to target priority families and genera that contribute to the GGI mission.
Fig. 2.
GGI–Gardens collection sheet for voucher and genetic samples.
Preparing living collections for genome tissue sampling
Internationally, Gardens hold an incredible amount of living plant diversity, including more than 450,000 taxa in more than 3300 Gardens (Botanic Gardens Conservation International, 2016). The collections are often identified to species level and may include rare taxa that are otherwise difficult to sample for genomic applications. A list of species in a living collection is extremely helpful to assist with indexing collected vouchers, identifying sampled tissues, and determining the original source of the material. If possible, sampling from a living collection can be optimized by identifying gaps in desired specimens and demarcating them in the garden before collecting. When possible, an experienced field botanist or member of the garden staff should accompany any sampling team to ensure collections are properly identified and appropriate material is collected in a timely fashion. Collections can be tracked through an organized note-taking system (see Fig. 2). GGI–Gardens has also replicated this collection sheet as a custom project in the iNaturalist web application environment (http://www.inaturalist.org).
Voucher specimen handling and collection
The term “voucher specimen” in this manuscript refers to a plant (or part of a plant) that has been collected, pressed, dried, labeled, mounted on archival paper, and deposited into a recognized herbarium (e.g., a member of Index Herbariorum [http://sweetgum.nybg.org/science/ih/]). The most important resources for sampling voucher specimens from a living collection are experience, time, and the proper tools. As with most botanical field collection methods, collectors should bring tools including, but not limited to, those listed and defined in Table 1. High-quality photographs are essential for GGI–Gardens collections to confirm determinations. Three photographs are typically taken, including (1) the specimen tag within the living collection that includes reference data for tracking provenance, (2) a photo of the specimen habit in the context of the living collections, and (3) a close-up photo showing detailed reproductive and vegetative material.
Table 1.
List of materials and their function for voucher specimen handling and collection associated with the GGI–Gardens project.
| Material | Function |
| Blotter | Thick, paper blotter sheets for absorption of moisture in plant press and drying oven. Can purchase from many herbarium supply vendors. |
| Camera | A high-quality camera for taking photographs of specimens. Both habit and close-up photographs are best. |
| Corrugate | Sheets of corrugated cardboard or aluminum are necessary to allow air to pass between specimens drying in a plant press. |
| Dryer | The most effective method to dry specimens collected in a plant press is using a dryer (45–50°C) for several days. After drying, specimens must be frozen (−80°C) for one week to kill any potential pests residing on the specimen. |
| Recognized herbarium | A recognized herbarium is essential for depositing collected herbarium vouchers. A searchable list of recognized herbaria can be found using Index Herbariorum (http://sweetgum.nybg.org/science/ih/). |
| Herbarium voucher | A pressed, dried, and mounted specimen, complete with a voucher label, typically mounted on 11.5 × 16.5″ archival herbarium paper. |
| Newsprint | A sheet of newsprint paper is used during the initial collection to separate each collected specimen. When specimens are mounted, they are removed from newsprint and affixed to archival herbarium paper. |
| Plant press with straps | Wooden slats are placed on the outside of arranged, collected plant specimens and tightly bound using two straps (high-weight cotton or nylon). Specimens are arranged inside of the press with a corrugate, blotter, specimen (in newsprint), blotter, corrugate, blotter, and so forth. |
| Pruning shears | Sharp cutting utensil for removing tissue from plant body. |
| Sterile solution | A solution of 10% bleach or 70% ethanol for sterilizing cutting utensils between specimen collections. |
| Torch (optional) | A butane or propane torch can be used to further sterilize collecting utensils between specimen collections. After rinsing pruning shears in sterile solution, they should be further sterilized by heating them for five seconds using a butane or propane torch. |
Genetic sample handling and collection
A genetic sample is collected at the same time a voucher specimen is prepared, but to preserve the intrinsic properties of DNA/RNA essential for genome sequencing, special handling and preparation methods are required. For a variety of reasons (summarized in Nagy, 2010), genomic DNA/RNA may degrade rapidly once removed from a living plant. Because of this, we recommend the preparation of two distinct genetic samples (a) silica-preserved and (b) liquid nitrogen–preserved. Chase and Hills (1991) and Corthals and Desalle (2005) discuss the biochemical and macromolecular merits of each preservation method, respectively, and outline infrastructural limitations for collectors. Many of the challenges for collections stored in liquid nitrogen are obvious, but the proximity of many Gardens to facilities that provide access to liquid nitrogen can mitigate these concerns. Together, GGI and GGBN can assist Gardens that wish to collect tissues from their living collections and preserve them for genomic research. A diagram showing recommended field collection equipment and resources is provided in Fig. 1, and a list is given in Table 2.
Table 2.
List of materials and their function associated with genetic sampling handling and collection with the GGI–Gardens project.
| Material | Function |
| Aluminum foil | Standard, cut into sufficient size to wrap cryogenic tube and protect barcode label. |
| Barcode labels | Provided by affiliated biorepository where specimens will be stored. |
| Biorepository | A facility that is a member of GGBN (contacted prior to collecting effort) that can advise collectors regarding specimen storage, curation, and preferred collection materials. |
| Cryogenic tubes | Plastic tubes with a rubber gasket on the cap used to store genome-quality tissues. New, disposable gloves should be used when filling the tube to prevent contamination of foreign DNA. A biorepository-approved barcode label should be affixed to the tube, and the tube should be wrapped in aluminum foil before placing it in liquid nitrogen. |
| Envelope for silica-dried specimen | An envelope is used to store plant tissues that will be preserved in silica gel. These envelopes should have a biorepository-approved barcode label affixed. The entire envelope should be placed into a resealable bag with silica gel and frozen when they are dry or soon thereafter. |
| Liquid nitrogen and transportation Dewar | Liquid nitrogen is used to flash-freeze genome-quality tissues from collected specimens. Liquid nitrogen is the best way to halt degradation of DNA and RNA and can be obtained from many vendors and often facilitated through a GGBN biorepository. Liquid nitrogen must be transported securely using a Dewar. Safety training should be completed prior to use and handling of liquid nitrogen. |
| Notebook with data sheet or electronic collection record keeping | Should include prepared data entry fields that carefully denote details of the collection including ecological and morphological notes for the specimen, number of samples taken, names of collectors, and the location. |
| Resealable bags (e.g., Ziploc bags) | Bags are used to hold collection materials. Tubes, envelopes, writing utensils, and silica gel should all be stored and transported in their own resealable bag. |
| Silica gel | Used for rapid desiccation of collected tissue to ensure high-quality preserved DNA. |
| Writing utensils | Pens, permanent markers, and pencils for writing under adverse conditions and ensuring notes and labels are permanent. The use of archival ink on silica gel envelopes is encouraged to ensure long-term viability of samples. |
Note: GGBN = Global Genome Biodiversity Network.
Biorepository handling and storage
Prepared genetic samples must be transferred to a biorepository promptly to prevent sample degradation and to ensure access for research applications. Biorepositories can be identified through the GGBN Data Portal (GGBN, 2011). Preparation and storage of genetic samples depend upon the ultimate destination and use of the samples. Each GGBN biorepository may have a different preference for storage based upon their capacity and infrastructure. For GGI–Gardens, collections are deposited into GGBN-affiliated biorepositories. Relevant legal and political considerations regarding data and material sharing are described in Seberg et al. (2016).
Common sampling problems and solutions
Insufficient material occurs when plants are small and/or reproductive material is limited. Some simple solutions include high-quality photographs and the use of older flowers or fruits as well as repeated visits to obtain the necessary material. Incorrect names are common because nomenclature (the naming of plants) is a living entity and therefore changes constantly; this may also occur as a result of staff error or quality control issues. In addition, Gardens have at their disposal a variety of nomenclatural verification materials with varying degrees of authority and accuracy. Errors in nomenclature can be easily corrected later when the data are being entered into the institutional names database. For taxonomic determinations, GGI–Gardens uses publications by experts and, as a back up, The Plant List (2013), which currently includes 17,020 genera in 642 families. The challenge of taxonomy is reflected in The Plant List by the “unassessed” status given to ca. 22.7% of species names for angiosperms alone; however, it is a widely accessible and up-to-date list of plant taxonomic names. GRIN (http://www.ars-grin.gov/) remains an important alternative resource for Gardens and other personnel for problematic unassessed taxon names. Misidentified plants are more common than we would like and more difficult to deal with. The use of an incorrect name for a collection means that later it may be misused in a research project and may lead to confusion during research applications. This problem can be minimized in two ways. First, determinations in Garden living collections are reliable, and mistakes usually result from collectors who misinterpret labels; it is therefore easier to confirm determinations with an expert. Second, we are currently adding barcode sequencing (Kress et al., 2005) to our workflow to validate taxonomic identifications and to add to the global barcode effort. Sample cross-contamination is a concern for any approach to sampling tissue for genomic research. Possible contaminants in a garden sampling workflow could be introduced by poor handling of material, insufficient protective equipment, and lack of sterilization methods. Our best practices outline sterilization methods using flame and the handling of equipment with disposable gloves to reduce the impact of contamination between samples. These handling techniques are common for many greenhouse procedures, but less common for botanical field collection; some practical methods have been outlined for orchids (Fitch, 2004).
Voucher preparation methods do not exist for living collections in Gardens, and guidance for genome-quality tissue collection has not been standardized for plant samples. Our protocol is based upon an adaptation of existing protocols that have been independently developed for genetic research. This protocol differs from alternatives because it ensures the long-term viability of genome-quality tissues by immediately flash-freezing in liquid nitrogen. It is important to note that liquid nitrogen is difficult to transport into the field, particularly for remote field sites; consequently, the availability of botanical diversity within Garden living collections facilitates a large-scale, organized effort to collect and preserve genome-quality tissues as we have outlined here. This represents a distinct advantage over traditional collection strategies that can present barriers to flash-freezing in liquid nitrogen in other contexts. Furthermore, GGI–Gardens collections contribute to a well-curated network of biorepositories that provide guidance documentation and develop best practices for physical genomic collections (Seberg et al., 2016). In addition to contributing fresh, genome-quality tissue to global biorepositories, this protocol also synergizes with Gardens that wish to voucher their collections and improve their accessibility and visibility for research but do not know how to begin. We considered alternative collecting materials for genomic applications (e.g., RNAlater; QIAGEN, Carlsbad, California, USA); however, the cost was preventative at our scale of collection when compared to readily accessible liquid nitrogen facilities (i.e., the GGBN-partnered NMNH Biorepository, Smithsonian Institution). This and many other GGBN-partnered biorepositories may provide assistance with access to liquid nitrogen for partnerships that contribute to GGBN (e.g., GGI–Gardens). Our data collection includes all available provenance data for living collections. However, one important disadvantage of our genome-quality tissue collection protocol is that material is not directly sourced from wild populations; however, we think that this protocol allows for unparalleled accessibility of material from across the plant tree of life.
CONCLUSIONS
Future problems and solutions are certain to arise, particularly with regard to rare or specialized living collections. In some cases, difficult-to-collect plants (e.g., palms) may require specialized collection procedures. Significant documentation exists for making vouchers from such plants, and a quick literature search will be of great help to Gardens. In many cases, the GGI–Gardens website (http://ggi.si.edu/ggi-gardens) will serve as an information repository for such best practices and to curate resources and documentation. As biobanks, DNA banks, and biorepositories emerge globally to address the research and infrastructural needs of comparative genomics research, a standard for sampling must be implemented to ensure consistency across samples used for high-impact genome-sequencing efforts. Several studies have attempted to establish a standard for plant DNA/RNA collection and preservation (e.g., Chase and Hills, 1991; Särkinen et al., 2012; Gaudeul and Rouhan, 2013; Neubig et al., 2014); however, none establish a workflow for tissue sampling that will contribute to a widespread genome biorepository network as we have here. We recommend this sampling workflow for GGI–Gardens partners, which will help to refine the optimal sampling protocol and standards that will define plant genome research.
Programs such as GGI–Gardens help Gardens show that their collections are critical to the future of scientific research. Furthermore, the availability of properly preserved leaf tissue for genomic work will greatly benefit downstream science. The workflow outlined here will facilitate a global sampling effort through voucher preparation at Gardens. Whether Gardens simply want to generate a set of voucher specimens from their collections or contribute genetic specimens for genetic or genomic research, we have provided a set of necessary steps to enable discovery from living collections. This list of resources will grow as the GGI–Gardens program continues to add international Garden partners. Membership in the GGI–Gardens consortium is available to interested Gardens that meet the criteria of the GGI–Gardens Memorandum of Cooperation (http://ggi.si.edu/ggi-gardens). Any questions can be addressed to the first or last author.
Appendix 1.
GGI–Gardens collection protocol.
A. Pre-fieldwork checklist/GGI–Gardens voucher collection equipment list:
Ensure that all of the following equipment is available and in good condition before you begin sampling.
- I. Voucher collection equipment
- a. Camera
- b. Butane torch
- c. Silica gel desiccant
- d. Envelopes for tissue dried in silica gel
- e. Printed adhesive barcode labels for liquid nitrogen cryovials and silica gel envelopes
- f. Pruning shears
- g. Newsprint
- h. Plant press
- i. Cryovials (8 mL) for liquid nitrogen
- j. Aluminum foil squares to wrap around cryovial
- k. Liquid nitrogen Dewar, full
- l. Printed collection sheets
- m. Pens, pencils, and markers
- II. Personal protective equipment (PPE) in the field
- a. Long pants
- b. Insect spray
- c. Sunscreen
- d. At least 1 liter of water
- e. Snacks and/or lunch
- f. Hat
- g. Appropriate clothing for weather (long pants, closed-toed shoes)
B. GGI–Gardens fieldwork protocol:
Use the following protocol for making collections in the field.
- I. Plant pressing
- a. Fill the entire sheet of newsprint. Refer to best practices guide for difficult or unusual plant specimens.
- b. Do not mix a pressed collection with multiple plants.
- c. Collect all important characteristics (e.g., flowers, fruit, and leaves if possible).
- d. Be sure to flip one leaf over so that the underside is showing.
- e. When splitting large collections into multiple sheets, do not label these multiple sheets “A, B, C…”; instead use a hyphen (–) after your collector number, followed by sequential numbers “1, 2, 3…”.
- II. DNA collection
- a. Test tubes for liquid nitrogen: Prioritize samples from leaves. Fill the test tube with as much leaf tissue as possible immediately after removing the tissue from the living specimen. Always place a printed barcode label onto the side of the tube and cover the filled, labeled tube with a square of aluminum foil before dropping it into the liquid nitrogen–filled Dewar.
- b. Envelope for silica gel: Cut leaf tissue into strips with sterilized pruning shears before putting into the envelope. Include an amount of leaf tissue at least as large as the area of the envelope itself. If needed, tear the leaf tissue into pieces so that it all fits, but do not overfill the envelope. On the outside of the envelope, write the collector name and accession number in archival ink, and attach the silica barcode sticker.
- c. Immediately add the corresponding circular barcode stickers to each collection data sheet in the appropriate place for liquid nitrogen and silica gel.
- d. Use young (but not too young), fresh, undamaged leaves.
- e. Sterilize each collection instrument with torch after each collection, whenever the cutting device will be used. Apply flame for 8–10 seconds to each cutting surface.
- III. Field inventory management (collection sheets)
- a. Fill out each empty line on the sheet.
- b. Include as detailed of a description as possible. Each detail counts!
- c. Always include a plant name. If you are not sure, ask a garden staff and/or use your smartphone (if you have one) to confirm the genus or family indicated on garden signs.
- d. If you can’t find a plant name, take a note of the camera image number and the collector number so that it can be identified quickly by an expert at the Museum before it is databased. Try to ID at least to plant family.
- e. Every time you stop to make a collection, note the specific location (e.g., greenhouse room number and section, display number or name, GPS coordinates, etc.).
- f. At the end of every day, write the next collector number on the next sheet; note in the margin that this number will be the next collection day.
- IV. Photographs
- a. Take three pictures:
- i. Label/tag that identifies the living collection from which a voucher is made, for tracking provenance.
- ii. Habit photo with the entire plant and some of the surroundings.
- iii. Close-up picture of vegetative and fertile material (flowers, fruit, spores of a fern, etc.).
- V. If a mistake is made
- a. Don’t panic!
- b. Note on the collection sheet what the mistake was and provide any corresponding information.
- c. If you forget to cover a liquid nitrogen cryovial with aluminum foil, void that barcode number and make a new collection.
- d. It’s always better to collect more than to miss a collection! Make an additional collection if you aren’t sure if you’ve collected sufficient material.
C. Post-collection specimen handling protocol:
After returning from the field, immediately complete the following steps.
I. Adjust the plant press in the drying room before bringing it upstairs. Be sure corrugates and blotter sheets are placed between each newsprint-bound specimen that is stored in the press.
II. Place Field Inventory collection sheets in the correct location so they can be databased as quickly as possible. Confirm that all collector numbers have a corresponding barcode sticker on the collecting sheet.
III. Place all photos onto a designated computer into a folder for GGI–Gardens. Upload pictures from your camera and record the photo file name/number in a spreadsheet that is linked with each collector number and barcodes for liquid nitrogen and silica. Once photos are transferred and renamed accordingly, feel free to delete them from the camera.
IV. Print or make copies of more collection data sheets if needed and write the next collector number on the first sheet for the following day.
V. Store all equipment in the designated place.
- VI. Carry the plant press upstairs to deposit in the dryer.
- a. Tighten the straps of the plant press.
- b. Ensure the press is placed in the dryer so that air can flow through efficiently. Boards should be arranged vertically, with the long side facing you. Air passes from the back of the dryer to the front.
- c. After ca. 10 days (depending on specimens) in the dryer, transfer specimens directly to the −80°C freezer for pest management. Store in a well-labeled box.
LITERATURE CITED
- Botanic Gardens Conservation International. 2016. Website http://www.bgci.org [accessed 12 February 2016].
- Chase M. W., Hills H. G. 1991. Silica gel: An ideal material for field preservation of leaf samples for DNA studies. Taxon 40: 215–220. [Google Scholar]
- Corthals A., Desalle R. 2005. An application of tissue and DNA banking for genomics and conservation: The Ambrose Monell Cryo-Collection (AMCC). Systematic Biology 54: 819–823. [DOI] [PubMed] [Google Scholar]
- Fitch C. M. 2004. The gardener’s guide to growing orchids. All-Region Guides, No. 178. Brooklyn Botanic Garden, Brooklyn, New York, USA. [Google Scholar]
- Fowler C. 2008. The Svalbard seed vault and crop security. BioScience 58: 190–191. [Google Scholar]
- Gaudeul M., Rouhan G. 2013. A plea for modern botanical collections to include DNA-friendly material. Trends in Plant Science 18: 184–185. [DOI] [PubMed] [Google Scholar]
- GGBN. 2011. (continuously updated). The GGBN Data Portal. GGBN Secretariat, NMNH, Washington, D.C., USA. Compiled by GGBN Technical Management, BGBM, Berlin, Germany. Website http://data.ggbn.org [accessed 12 February 2016].
- Glenn T. C. 2011. Field guide to next-generation DNA sequencers. Molecular Ecology Resources 11: 759–769. [DOI] [PubMed] [Google Scholar]
- ISBER. 2012. 2012 Best practices for repositories. Collection, storage, retrieval, and distribution of biological materials for research. Biopreservation and Biobanking 10: 79–161. [DOI] [PubMed] [Google Scholar]
- Kress W. J., Wurdack K. J., Zimmer E. A., Weigt L. A., Janzen D. H. 2005. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences USA 102: 8369–8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z. T. 2010. A hands-on overview of tissue preservation methods for molecular genetic analyses. Organisms, Diversity & Evolution 10: 91–105. [Google Scholar]
- Neubig K. M., Whitten M. W., Abbott R. J., Elliot S., Soltis D. E., Soltis P. S. 2014. Variables affecting DNA preservation in archival plant specimens. In W. L. Applequist and L. M. Campbell [eds.], DNA banking for the 21st century. Proceedings of the U.S. Workshop on DNA Banking in St. Louis, Missouri, 2014, 81–136. William L. Brown Center at the Missouri Botanical Garden, St. Louis, Missouri, USA. [Google Scholar]
- Särkinen T., Staats M., Richardson J. E., Cowan R. S., Bakker F. T. 2012. How to open the treasure chest? Optimising DNA extraction from herbarium specimens. PLoS ONE 7: e43808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seberg O., Dröge G., Barker K., Coddington J. A., Funk V., Gostel M., Petersen G., Smith P. P. 2016. Global Genome Biodiversity Network: Saving a blueprint of the tree of life—A botanical perspective. Annals of Botany 118: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner D. M., Ruess H. Curating DNA specimens. In W. L. Applequist and L. M. Campbell [eds.], DNA banking for the 21st century. Proceedings of the U.S. Workshop on DNA Banking in St. Louis, Missouri, 2014, 71–80. William L. Brown Center at the Missouri Botanical Garden, St. Louis, Missouri, USA; 2014. [Google Scholar]
- The Plant List. 2013. Version 1.1. Website http://www.theplantlist.org/ [accessed 12 February 2016].
- Thiers B. 2016. (continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Website http://sweetgum.nybg.org/science/ih/ [accessed 12 February 2016].
- van Slageren M. W. 2003. The Millennium Seed Bank: Building partnerships in arid regions for the conservation of wild species. Journal of Arid Environments 54: 195–201. [Google Scholar]
- Wong P. B., Wiley E. O., Johnson W. E., Ryder O. A., O’Brien S. J., Haussler D., Koepfli K.-P., et al. 2012. Tissue sampling methods and standards for vertebrate genomics. GigaScience 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimkus B. M., Ford L. S. 2014. Genetic resource collections associated with natural history museums: A survey and analysis to establish a benchmark of standards. In W. L. Applequist and L. M. Campbell [eds.], DNA banking for the 21st century. Proceedings of the U.S. Workshop on DNA Banking in St. Louis, Missouri, 2014, 9–44. William L. Brown Center at the Missouri Botanical Garden, St. Louis, Missouri, USA. [Google Scholar]